metamorworks/iStock via Getty Images
I first became aware of Passage Bio (NASDAQ:PASG) as a competitor to Sio Gene Therapies (SIOX), a company about which I have written extensively. Previously, the market cap and enterprise value of PASG were much too large relative to SIOX for me to consider an investment, but in this brutal biotech market, that has now changed.
bigcharts.marketwatch.com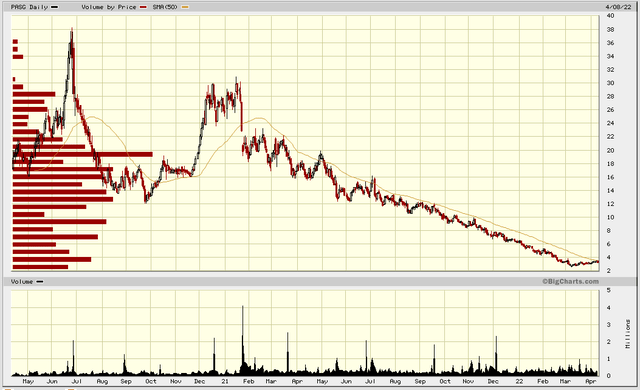
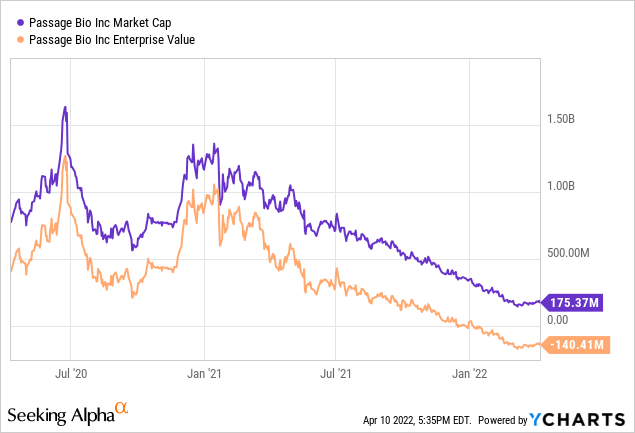
The company is now trading at an EV of negative $140M, which implies that currently the market believes that the company’s future work will result in the destruction of 140 million dollars. Contrast that with 18 months ago, when the market was valuing future progress at a positive $1.2B!
These are the swings of investor sentiment which often overshoot in both directions. So with that in mind, let’s see what the company currently offers.
Pipeline
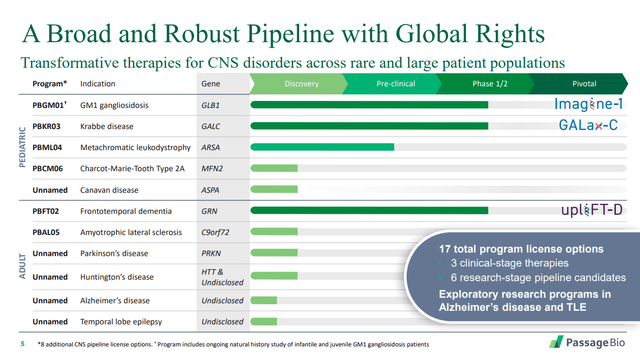
The company has three Phase 1/2 studies in progress, two in pediatric indications and one in an adult indication.
Source: Passage Bio
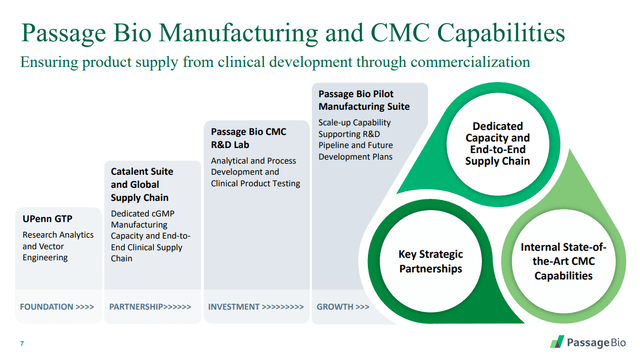
We look at each of these below, but before doing so, consider also its manufacturing capabilities:
Source: Passage Bio
1. GM1 Gangliodosis
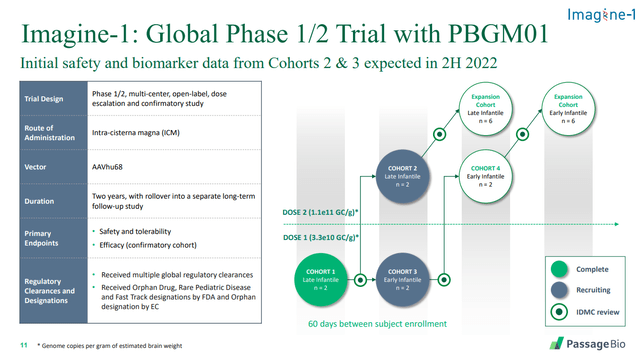
PASG is early in its gene therapy trials for GM1 gangliodosis. Here’s the trial design including enrolment status.
Source: Passage Bio
Given that SIOX seems well ahead of PASG in this indication, I think that PASG will slow-roll this and focus on its other programs instead. That’s particularly true in light of the company’s announcement of making changes to reduce cash burn (which I discuss below).
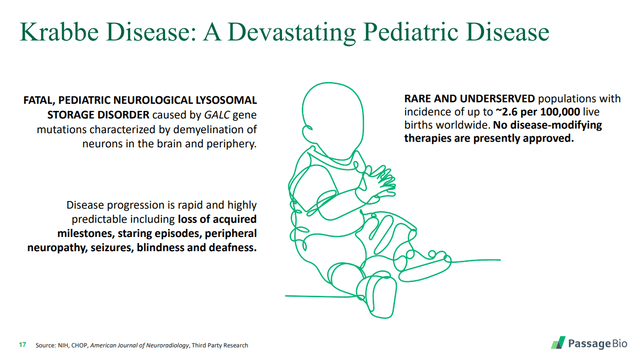
2. Krabbe Disease
Krabbe disease is a rare but devastating genetic pediatric disease in which the myelin of nerves in the brain is destroyed. According to the Mayo Clinic:
In most cases, signs and symptoms of Krabbe disease develop in babies before 6 months of age, and the disease usually results in death by age 2. When it develops in older children and adults, the course of the disease can vary greatly.
There’s no cure for Krabbe disease, and treatment focuses on supportive care. However, stem cell transplants have shown some success in infants who are treated before the onset of symptoms and in some older children and adults.
Krabbe disease affects about 1 in 100,000 people in the United States. It is also known as globoid cell leukodystrophy.
As shown in the slide below, there are currently no disease modifying therapies to treat this devastating disease.
Source: Passage Bio
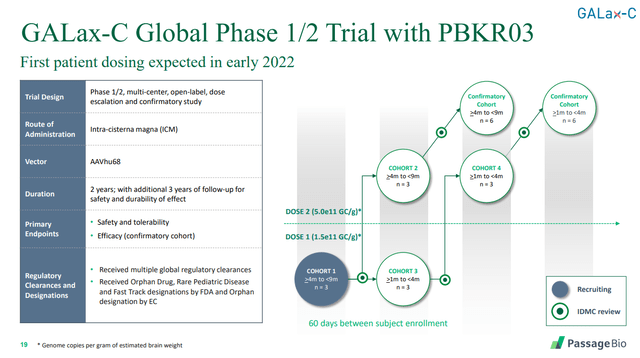
PASG’s approach to treat this disease is to introduce functional genes to produce GALC. The initial clinical trial design is as follows:
Source: Passage Bio
Personally I think this is the company’s most promising avenue, and it’s the one that I’ll be watching most closely to decide whether or not to invest in the company.
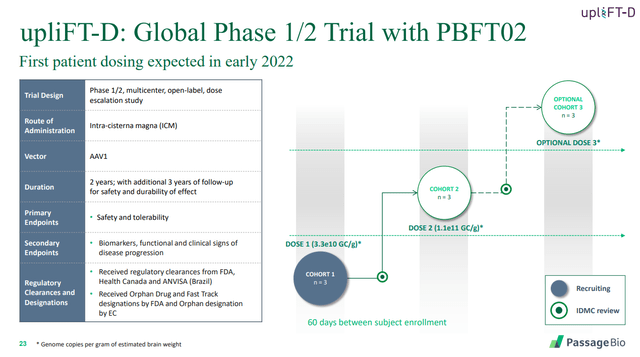
3. Frontotemporal Dementia – GRN
This rare but devastating disease, which affects a full gamut of brain functions is also caused by genetic mutations, in particular in the GRN gene. The mechanism is not yet well understood, but here’s the best description I’ve found, courtesy of Medlineplus (with my emphasis):
GRN-related frontotemporal lobar degeneration results from mutations (pathogenic variants) in the GRN gene. This gene provides instructions for making a protein called progranulin. Progranulin is active in many different tissues in the body, where it helps control the growth, division, and survival of cells. Progranulin’s function in the brain is not well understood, although it appears to play an important role in the survival of nerve cells (neurons).
Most mutations in the GRN gene prevent any progranulin from being produced from one copy of the gene in each cell. As a result, cells make only half the usual amount of progranulin. It is unclear how a shortage of this protein leads to the features of GRN-related frontotemporal lobar degeneration. However, studies have shown that the disorder is characterized by the buildup of a protein called TAR DNA-binding protein 43 (TDP-43) in certain brain cells. The TDP-43 protein forms clumps (aggregates) that may interfere with cell functions and ultimately lead to cell death. Researchers are working to determine how mutations in the GRN gene, and the resulting loss of progranulin, are related to a buildup of TDP-43 in the brain.
The features of GRN-related frontotemporal lobar degeneration result from the gradual loss of neurons in regions near the front of the brain called the frontal and temporal lobes. The frontal lobes are involved in reasoning, planning, judgment, and problem-solving, while the temporal lobes help process hearing, speech, memory, and emotion. The death of neurons in these areas causes problems with many critical brain functions. However, it is unclear why the loss of neurons occurs in the frontal and temporal lobes more often than other brain regions in people with GRN-related frontotemporal lobar degeneration.
PASG’s approach here is once again to deliver a functional GRN gene to produce progranulin. As shown in the trial design slide below, PASG hopes to dose the first patient in 2022.
Source: Passage Bio
Cash and Cash Burn
Given that all of the company’s programs are in very early stages, cash on hand and cash burn are critical parameters, particularly in today’s biotech environment where no one is ready to fund early stage research.
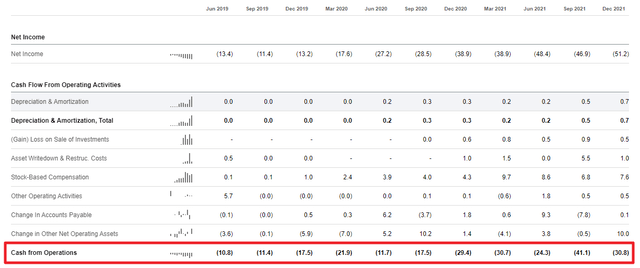
PASG had been burning about $30M per quarter as shown in SA’s very helpful summary tables:
Source: Seeking Alpha
However, on March 15th, 2022, PASG acknowledged the current biotech environment by announcing its intention to cut 13% of its workforce and to focus only on its three lead indications (i.e. the three I discussed above).
This action has the effect of extending the company’s cash runway into early 2024.
Upcoming Milestones
The same announcement included a list of milestones to expect in the near term. These are:
- Dose first patient in Phase 1/2 study for FTD-GRN in early 2022. Additional clinical data milestone timing to be provided following dosing of first patients.
- Submit Investigational New Drug application for Phase 1/2 clinical program for PBML04 (metachromatic leukodystrophy) in mid-2022.
- Present interim safety and biomarker data for Cohorts 2 (late infantile, high dose) and 3 (early infantile, low dose) for Imagine-1 clinical trial for GM1 in 2H 2022.
- Present interim safety and biomarker data for Cohort 1 for GALax-C clinical trial for Krabbe disease by YE 2022.
Conclusion
Though I’m currently on sidelines, and still think SIOX is the better buy at today’s stock prices, I’m closely watching PASG’s progress in Krabbe disease and FTD-GRN to potentially buy in to the stock. On a more general level, I will also be following the company’s progress to better understand the gene-editing space overall. I hope to provide additional updates as warranted by the company’s progress.


Be the first to comment