Artur Plawgo
INmune Bio (NASDAQ:INMB) has two treatments in clinical trials, both work on the assumption that chronic low-grade inflammation is behind many ailments, like AD (Alzheimer’s Disease) and TRD (treatment-resistant depression). It has two drugs in clinical trials, from the latest company IR May 2022 presentation:
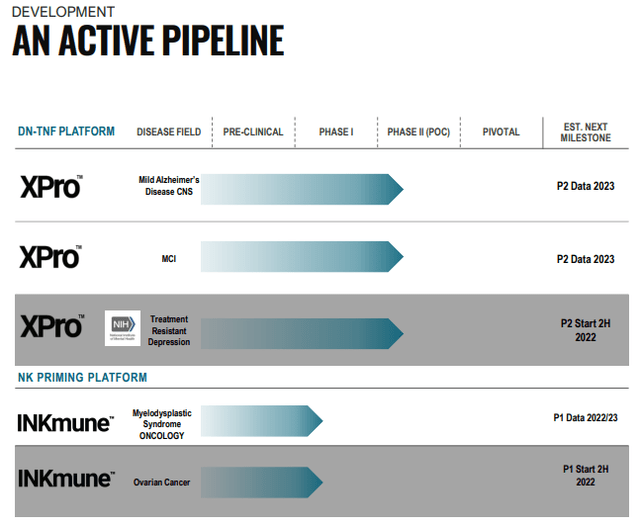
INMB IR presentation
The company has been buffeted to some degree by the FDA for a change in manufacturing their main drug, XPro, leading to a clinical hold letter from The FDA. From the Q2CC:
The new XPro was manufactured by KBI at their Boulder facility, a different manufacturing site than the previous XPro batches. The triple challenge of the new company manufacturing a first-in-class therapy at a new manufacturing site, all contributes to the FDA’s caution. We are working with the FDA to answer their CMC questions.
This is leading to trial delays but management expects these issues to be resolved although it is taking a surprising amount of time already. Investors need to keep in mind that this is a manufacturing problem, it doesn’t have anything to do with the effectiveness of XPro or the design of the clinical trials.
And there is some way around this as they continue enrollment of patients for two clinical tests (one, AD02 for mild AD and the other one, AD03, for mild cognitive impairment) in Australia, where they are not on hold
XPro
According to management, neuroinflammation is likely to be a major cause of neurodegenerative diseases like AD. The company’s drug, XPro, functions as a selective TNF inhibitor which only inhibits the bad TNF or sTNF (soluble TNF). From the company IR May 2022 presentation:
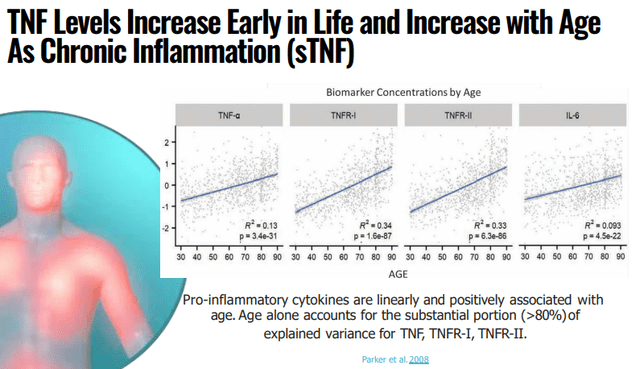
INMB IR presentation
And there is significant evidence that sTNF plays an important role in causing AD:
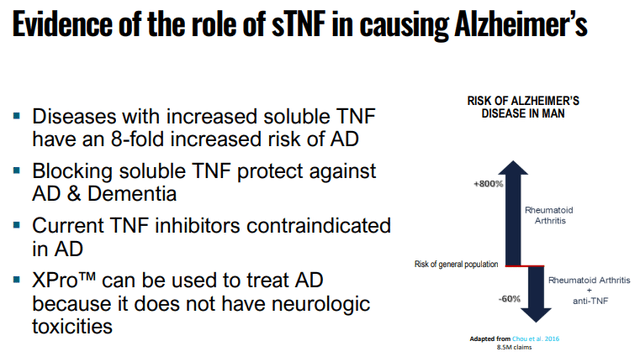
INMB IR presentation
XPro has a number of advantages versus traditional TNF inhibitors, which are already on the market:
- XPro is not an immunosuppressant, it is not inhibiting the good TNF
- It does not lead to demyelination, in fact, it promotes remyelination
- It is neuroprotective
- It does enhance neuroplasticity
- It can be administered subcutaneously (not need to be administered perispinally).
In essence, from the September 2022 IR presentation:
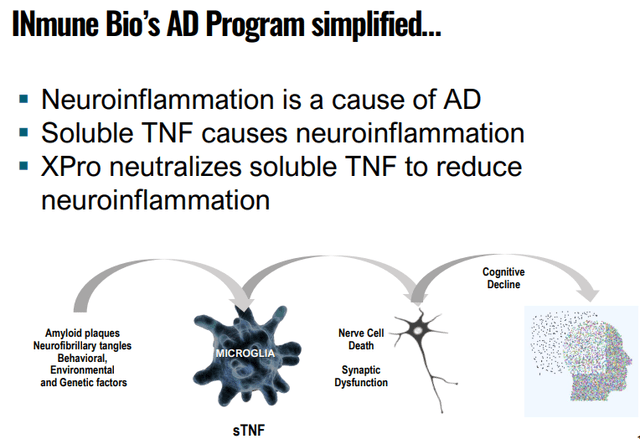
INMB IR presentation
Preclinical testing has shown ample effectiveness. The Phase 1 study showed a marked improvement in a host of biomarkers, see slides 11-18 of the linked May/22 IR presentation, and the most important ones are summarized in their November 2021 IR presentation:
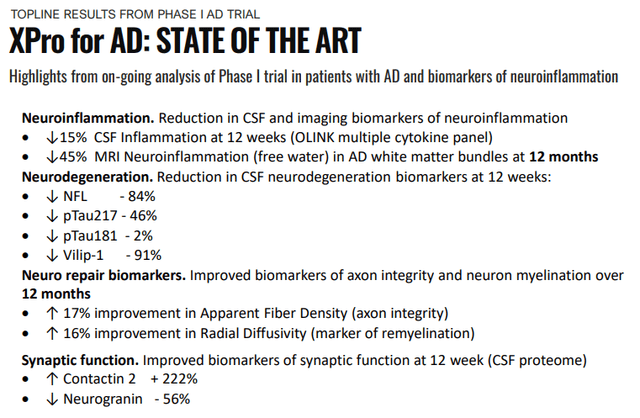
INMB IR presentation
These were pretty stunning improvements after just 12 weeks, summing things up (Company PR):
“The data from this Phase I trial show that XPro™ decreases biomarkers of neuroinflammation and nerve cell death while improving biomarkers of neurorepair in patients with AD,” stated RJ Tesi, M.D., Chief Executive Officer of INmune Bio. “Now we can add improvements in neuroimaging biomarkers of remyelination to the beneficial effects of XProTM in these patients.
As many of these biomarkers appear well before any cognitive symptoms, this also enables the prediction of the progression from MCI (mild cognitive impairment) to AD.
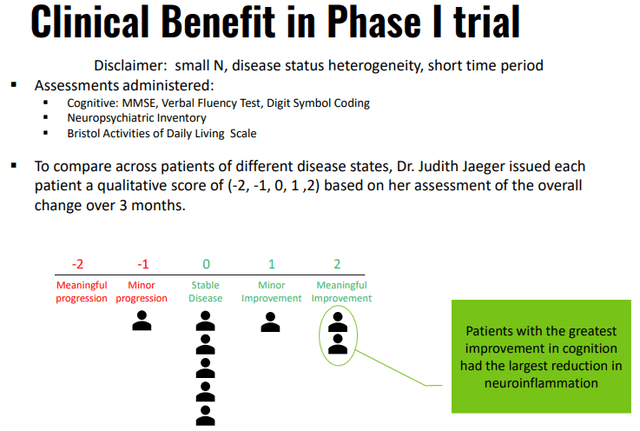
INMB IR presentation
There were also a few very good testimonials of the few patients in the P1 trial with two out of six patients being able to go back to normal function, see the ‘Testimonials’ section in the excellent article by SA colleague E. Roudasev.
Then there is this (Q3CC):
Yet another nationwide study has reported that TNF inhibitors reduce the risk of dementia. There are now 5 epidemiological studies examining more than 60 million records that show anti-TNF therapies reduce the risk of AD and/or dementia by up to 75%.
So at the minimum, XPro is a very promising drug for treating AD, but it could also open a whole new field for other inflictions that are likely to be caused by chronic inflammation.
Treatment-resistant depression
One of these is TRD, treatment-resistant depression (defined as people who do not respond to two or more therapies), which afflicts 33% of people who suffer from depression, or some 7M people. Here too, there is compelling evidence that XPro can work, from the company website:
Although there are many inflammatory factors involved in depression, Tumor Necrosis Factor (TNF) may play a unique role in TRD. TNF is reliably increased in TRD patients and TNF levels are associated with the number of failed treatments and poorer treatment outcomes5. Anti-TNF therapy has been shown to improve mood in patients suffering from inflammatory diseases such as psoriasis6 and Crohn’s disease4. Finally, a proof of concept clinical trial demonstrated that blocking TNF improved mood in a subset of TRD patients that had elevated biomarkers of inflammation (c-reactive protein; CRP)7. The last point is important. Elevated CRP in the blood is a biomarker predicting TRD in patients with depression suggesting that a simple and widely available laboratory test could change the management of patients with TRD8.
So equally promising and in fact, XPro could be used for a host of neurodegenerative diseases like Parkinson’s, MS, and others. The company recently received a patent for treating CNS (Central Nervous System) diseases with XPro.
P2 trials
The company has separated trials for mild AD and MCI (mild cognitive impairment) whereas the competition combines these into a single trial for dearly AD, which (Q2CC):
is an artificial construct or combination of mild Alzheimer’s disease plus MCI. We believe two trials is more informative and less risky.
The company is using EMACC (a purpose build cognitive test) as the primary cognitive endpoint.
Given the very promising results over a relatively short period the phase 2 trials can also be short:
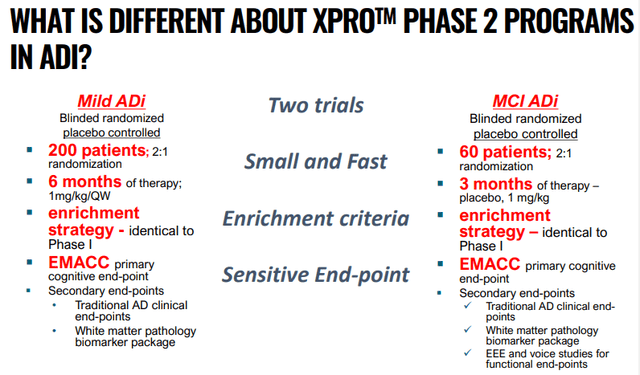
INMB IR presentation
The start has been upended by the FDA requesting additional manufacturing data, but the enrollment of patients continues in Australia and management thinks it can clear this up fairly soon.
The results are now expected in late 2023 or early 2024 and that won’t be the end of it as the company will have to do a P3 trial after that.
There are patients from the P1 trial who have requested a continued supply of XPro and they will be monitored as well.
These patients are technically not part of the trial as none of them are on a placebo and it’s not a double-blind procedure, but the advantage (besides the compassionate use) is that it generates additional data over an extended period of time, which is likely to bolster claims about the long-term safety profile of XPro.
One might also surmise that since these patients themselves have requested continued use (supported by their physicians), they are noticing the benefits of XPro.
The company will also initiate a P2 study for TRD (treatment-resistant depression) funded by a $2.9M NIH grant upon FDA hold resolution.
INKmune
INKmune is a natural killer cell priming platform for solid tumors (90% of all cancers, while cell therapies tend to focus on liquid tumors). INKmune works by priming a patient’s NK cells (killer cells) to destroy left-over cancer cells can prevent the re-emergence of cancer. From the company website:
The NK cells kill target cells by two mechanisms, first by secretion of perforin molecules which create holes in the cancer cell membrane and allow entry of NK-cell secreted enzymes called “granzymes” into the cancer cell, which kills the cell. This is called “NK cell degranulation”. The second killing mechanism is by binding to “death receptor” molecules on the surface of the cancer cells and signalling to the cell to commit suicide by a process called “apoptosis”.
However, in some patients, cancer cells evade detection by the innate immune system (NK cells) and this causes cancer to relapse. The company has developed a model of the signals that activate the NK cells (company website):
INmune Bio has created a therapeutic product that can be infused into the patient to move the resting NK cells to primed state without initiating NK cell degranulation. We call this product INKmune™… Importantly, we have shown that INKmune™ primed NK cells form much stronger immune synapses with cancer cells and this is essential to deliver the lytic payload and death receptor signals to cancer cells which are resistant to resting NK cells.
And from the Q2CC:
The increased fitness of INKmune primed NK cells is due to an upregulation of mitochondrial proteins. I remind you that mitochondria, the engines of the cell are essential for NK survival and we believe this increased fitness may be the cause of the extended therapeutic persistence in INK – of memory-like NK cells and INKmune treated patients.
Or from the Q3CC:
immune converts patients normal resting NK cells into what are called memory-like NK cells. — and that these target solid tumors even in the presence of immunosuppressive immunoregulatory cells and extreme hypoxia that as I’ve said, we see in the TME.
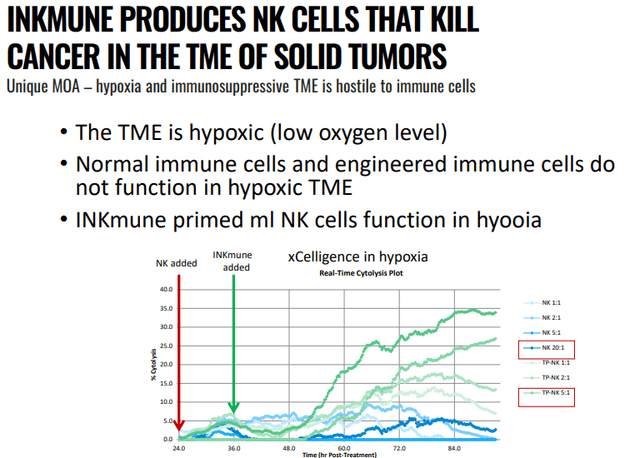
INMB IR presentation
TME stands for active tumor microenvironment where the presence of immunosuppressive regulatory cells and the low levels of oxygen immune response (hypoxia), and NKImune has been able to do that in a wide variety of in-vitro settings with solid tumors from ovarian cancer, breast cancer, prostate cancer, renal cell carcinoma, and nasopharyngeal cancer cells.
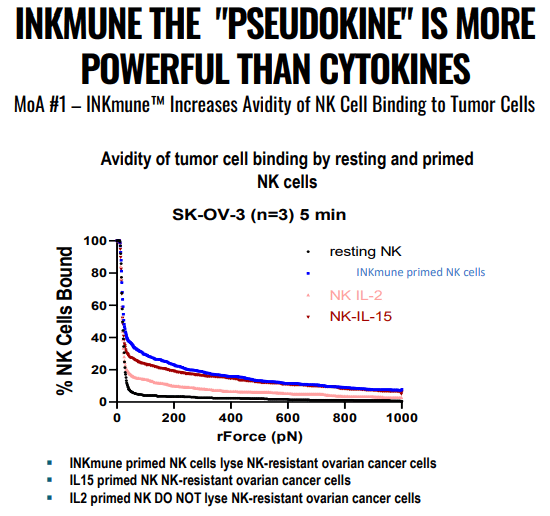
INMB IR presentation
It acts as a cytokine cocktail, making the patient’s own NK cells better as it improves avidity (tumor binding) in vitro, the worse avidity, the shorter the survival.
Unlike alternative priming treatments like Rituximab, Herceptin, IL-2, IL-15, and IL-12, INKmune is not antigen-specific so there is no need to identify the specific tumor antigen and there are no signs it is targeting non-cancerous cells (which is a problem with some of the alternatives).
It’s cheap (unlike the alternatives), manufacturable in bulk, available off the shelf, and can be given to patients on an outpatient basis. See website and a short video, or even the full linked presentation. In short:

INMB IR presentation
Phase 1 trial
INKmune small ongoing P1 trial consists mostly of compassionate use cases which limits data gathering (no placebos, not double-blind). It treated 4 patients.
One patient with MDS (myelodysplastic syndrome) and three with a more severe disease AML (acute myeloid leukemia), were all extremely ill. The trial is still ongoing, but there are some preliminary results:
- The infusions are well tolerated, with no side effects in any of the four patients.
- All patients showed evidence of NK cell activation.
From the Q2CC:
to our knowledge, INKmune priming is the only INKmune – NK therapy reporting improved functional mitochondrial function and therapeutic persistent lasting more than a hundred days.
The first patient is a 78-year-old male patient with severe MDS and a very poor quality of life. He remains alive 15 months after the first treatment (three treatments have been given to the patients at the time of the Q2CC) and his quality of life has improved dramatically (he now plays badminton with his friends and he no longer needs frequent platelet transfusions).
The second, a high-risk 20-year-old female initially was also successful (she was even discharged from the hospital), from the Q2CC:
Within one month her neutrophil count improved significantly and sufficiently for her to be discharged home for the first time in seven months, the disease burden reduced and she went from mixed donor chimerism to full donor chimerism. But to put that in simpler terms, her donor stem cell graft initially did not fully establish itself. And after the immune therapy, it did. Her disease became controllable with routine chemotherapy and seven months after completing INKmune treatment, she remained alive and with control disease and was awaiting a suitable donor for a second transplant.
However, she still died as the disease relapsed and she wasn’t responsive to chemotherapy. The third patient was in an even less promising state (Q2CC):
Our third patient who followed very shortly after the second patient was a young man in his late teenage years, who also had relapse acute myeloid leukemia, but he’d actually had two flailed allogeneic stem cell transplants. So was even higher risk than the young lady. He had a very high level of disease in his bone marrow, including bone marrow pain or bone pain and he was unresponsive to chemotherapy.
This patient did show a significant activation of NK cells after infusions but these never developed the memory-like phenotype seen in other patients. He is still alive with the help of chemotherapy.
Recently a fourth patient was added under compassionate care. She also is a very ill young AML patient and showed evidence of improved NK cell functions and remains well with a very low level of detectable disease. One should keep in mind:
- Only the lowest dose of INKmune has been given to all.
- These are very sick patients who were subject to alternative treatments that didn’t work.
- Three out of four patients are compassionate care patients.
The initial indications are promising, but given the nature of the patients and the small scale, it is of course way too early for any definite conclusions. The trial will conclude in H1/23.
Additional trials in Southampton UK for ovarian cancer have already been approved and two in the EU for MDS are already submitted and others are planned for other solid tumors.
In summary, we can say that early results are very promising but a lot of work remains ahead. This too is a multi-billion dollar market opportunity.
INB03
INB03 is a drug that can change the tumor microenvironment (TME) to improve the response to targeted therapies for cancer. It’s in the pre-clinical stage still and there was little news to report, from the Q2CC:
presented data at the AACR Conference and HER2+ Targeted Therapy Summit showing INB03’s ability to reverse Mucin 4 (MUC4) expression, a negative predictor of treatment success, in HER2 breast cancer cell line (JIMIT-1) to re-establish sensitivity to trastuzumab and tyrosine kinase inhibitors.
With potential blockbusters like XPro and INKmune further advanced, most focus should be on these for investors, but it’s good to know they have additional candidates.
Finances
Some relevant data, the company doesn’t yet produce revenue:
- Net loss down from $9.5M to $7.7M y/y
- R&D $5.2M, down from $6.5M y/y
- G&A $2.4M down from $2.5M y/y
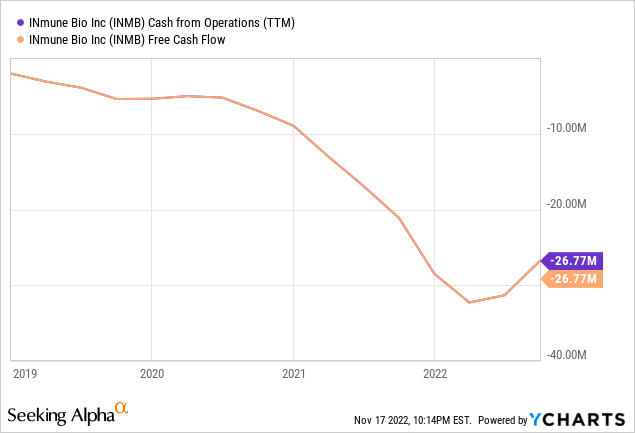
- Cash $57.4M (down from $61.2M at the end of Q2) sufficient through FY23
- Forex tailwind (AUS$) and Australian rebates
Cash outflow has stabilized at a little over $30M a year so they should indeed be good until at least the end of FY23:
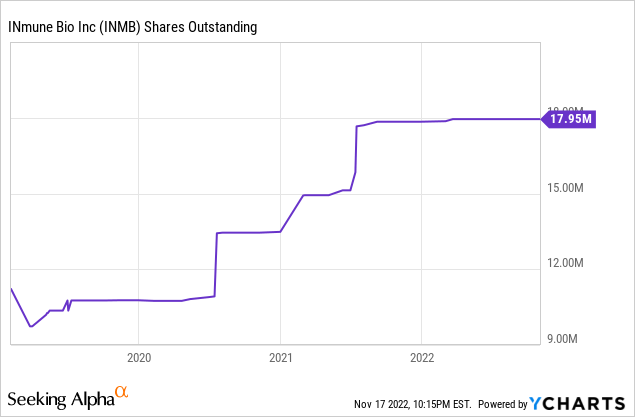
So the share count is likely to be fairly stable for a year and a half:
Upcoming milestones
- The P2 XPro trials are largely on hold because FDA but the AD02 trial is still enrolling patients in Australia, which is actually cheaper due to the strong dollar and Australia’s rebate program.
- After the resolution of the FDA issue the three-month 60 patient P2 for mild cognitive impairment will resume with results expected mid to H2/23.
- Initiate P2 XPro trial treatment-resistant depression depending on FDA resolution and financed in part with a $2.9M NIH grant.
- Additional open-label Phase 1 data from the ongoing P1 trial for INKmune in high-risk MDS/AML are expected later this year.
- Launch a second INKmune trial in a solid tumor Phase 1 program with a target date sometime early 2023.
And further down the horizon, there are other possible indications for both INKmune and XPro:
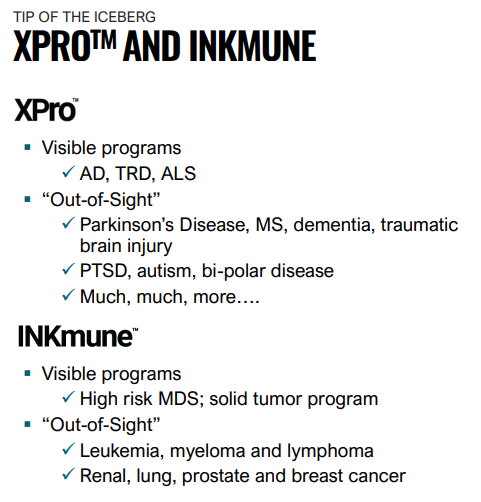
INMB IR presentation
Valuation
With 18M shares and another 4.9M from options (and a few from warrants), the company has almost 23M shares fully diluted for a market cap (at $7 per share) of $160M and an EV of $100M. This is a fraction of the potential, given the possibilities, especially of XPro.
Conclusion
XPro is a very promising drug with multiple use cases serving extremely large markets, it could be a blockbuster. Keep in mind that success is never guaranteed but there are multiple promising signs and trial results.
INKmune could also be a blockbuster with multiple use cases but it is less advanced and showed promising results in a very small trial with patients that had tried everything else.
They also have a few other drug candidates like INB03, but that’s still pre-clinical. The near-term catalyst would be a resolution of the manufacturing issues which will allow the company to resume its clinical trials.


Be the first to comment