kgtoh/iStock via Getty Images
Once again, Eisai (OTCPK:ESALY) and Biogen (BIIB) are touting success for an anti-amyloid drug that has limited efficacy combined with safety issues. The first and most important point to make about lecanemab (formerly BAN2401) is that it has almost no effect on non-APOE4 carriers. An earlier phase 2 clinical trial produced the following results:
On the ADCOMS… where the highest dose group declined 30 percent less than placebo, APOE4 carriers declined 63 percent less than placebo and non-carriers only 7 percent less (source).
In terms of CDR-SB scores, patients on lecanemab in this trial declined by an average of 26 percent less versus placebo. In the recent phase 3 Clarity trial the slower rate of decline in CDR-SB scores was 27 percent. No distinction was made in terms of progression of the disease between APOE4 carriers and non-carriers as measured by CDR-SB scores for either trial, but one can safely assume that lecanemab slowed down the rate of progression magnitudes more for APOE4 carriers than non-carriers using this measure.
The lesson for the FDA is that lecanemab is not effective for non-carriers and should not be approved for this group.
The question is whether, the FDA should approve lecanemab for APOE4 carriers. The following chart helps to get at the efficacy question.
(Alzforum)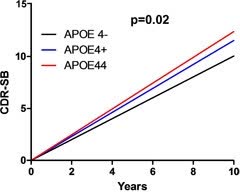
ApoE4 carriers with mild AD progress faster than non-carriers (article).
The mean baseline CDR-SB score for the Clarity trial was 3.2 which is right above the borderline between late mild cognitive impairment and very mild Alzheimer’s disease (Table 3). On average, individuals with late mild cognitive impairment advance .75 points over 18 months (the length of the Clarity trial) while those with mild Alzheimer’s disease advance 2.1 points over the same time period (extrapolated from one year progression). If half the participants in the Clarity trial had late mild cognitive impairment and the other half had (very) mild Alzheimer’s disease, the average decline at 18 months would be around 1.4 points. But those without the APOE4 gene might progress by about .7 points on average, whereas the average for APOE4 carriers might be closer to 2.1 points. So the non-APOE4 group may have progressed from a baseline of 3.2 to 3.9 points whereas the APOE4 group may have progressed from 3.2 to 5.3 points. The slope in the chart above suggests that these rough calculations are not too far off.
Eisai and Biogen reported a slower decline of .45 points (on average) in CDR-SB scores in the Clarity trial. If all of this reduced decline was in the APOE4 group, then APOE carriers would have declined be .9 points less, which means that they would decline by 4.4 points instead of 5.3 points. By contrast, those without the APOE4 gene would still decline to 3.9 points at 18 months. For some APOE4 carriers at least, this would represent a clinically significant slowing down in the progression of the disease (discussion).
The second issue involves safety. Because it preferentially pulls out oligomers rather than larger plaques, lecanemab has a lower risk of brain bleeding and swelling than anti-amyloid drugs which primarily remove plaques. Eisai and Biogen reported that the percentage of trial participants on lecanemab who were symptomatic for these side effects was in the low single digits (press release). However, brain bleeding and swelling can result in death. So the question is whether modestly slowing down the progression of mild Alzheimer’s disease in APOE4 carriers is worth the risk.
If the FDA approves lecanemab for all patients with mild Alzheimer’s disease, Medicare should then restrict coverage to only APOE4 carriers, if it provides coverage at all.
As a brief but important aside, it is important to understand why only APOE4 carriers benefit from treatments that remove amyloid oligomers. Amyloid oligomers are a secondary factor in Alzheimer’s disease, remove all or nearly all oligomers, the factors that originally triggered the disease remain. In APOE4 carriers amyloid levels are high enough to add to already existing levels of oxidation and nitration, which damage key enzymes, receptors, and transport systems in the brain (study). To go from slowing down the progression in APOE4 carriers to largely stabilizing Alzheimer’s disease in all mild Alzheimer’s disease patients, oxidative and nitrostative mediated damage has to be at least partially reversed (example).
The Clarity trial does add some clarity to our understanding of Alzheimer’s disease. Unlike, Eisai and Biogen’s trial for aducanumab/Aduhelm where you had two different sets of somewhat contradictory data, Eisai and Biogen have now produced a single-stream trial which met all its endpoints. The trial also adds to our understanding of which groups benefit to some degree from anti-amyloid treatments (APOE4 carriers) and which groups do not (non-APOE4 carriers).
For aducanumab/Aduhelm, Eisai and Biogen initially went from everything (approved for all patients at all stages of Alzheimer’s disease) to nearly nothing (Medicare coverage only for those in clinical trials for Aduhelm). This time, I think the FDA or more likely Medicare will at the very least restrict access to lecanemab to APOE4 carriers. In a sense, that would still be a triumph for Eisai and Biogen and for the amyloid hypothesis for Alzheimer’s disease, but a qualified triumph. Certainly, it would not validate the unbridled praise that those most invested in the amyloid hypothesis of Alzheimer’s disease have heaped upon lecanemab. But it would still represent a substantial market for Eisai and Biogen. Given that expectations are now set very high, there might be at least a slight sell-off of Eisai and Biogen stock if their drug candidate is restricted to mild Alzheimer’s disease patients who are APOE4 carriers.


Be the first to comment