Godji10
A Quick Take On Third Harmonic Bio
Third Harmonic Bio (THRD) has filed to raise $150 million in an IPO of its common stock, according to an S-1 registration statement.
The firm is a clinical-stage biopharma developing treatments for dysfunctional mast cell activity disorders.
THRD is seeking to further develop intellectual property licensed from Novartis.
I’ll provide a final opinion when we learn more IPO details from management.
Third Harmonic Overview
Cambridge, Massachusetts-based Third Harmonic was founded to develop KIT inhibition capabilities in an oral small molecule drug to treat conditions such as urticaria (hives), asthma and gastrointestinal conditions.
Management is headed by Chief Executive Officer Natalie Holles, who has been with the firm since August 2021 and was previously president and CEO of Audentes Therapeutics and prior to that was Senior VP, Corporate and Business Development at Hyperion Therapeutics.
The firm’s lead candidate is THB001 and is in Phase 1 trials for dermatologic and respiratory condition treatments.
Management expects to report results from its Phase 1b proof-of-concept trial for treating chronic inducible urticaria by the second half of 2023.
Below is the current status of the company’s drug development pipeline:
Company Pipeline (SEC EDGAR)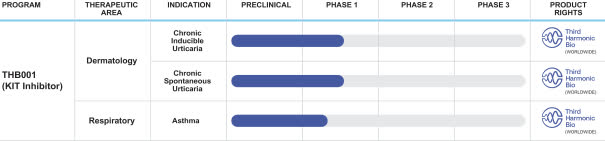
Third Harmonic has booked fair market value investment of $170.2 million as of June 30, 2022, from investors including Atlas Ventures, BVF Partners, General Atlantic, Novartis (NVS), and OrbiMed.
Third Harmonic’s Market & Competition
According to a 2022 market research report by Technavio, the global market for treating various types of urticaria is forecast to increase in value by $1.3 billion from 2021 to 2026.
This represents a forecast CAGR (Compound Annual Growth Rate) of CAGR of 10.35 over the study period.
Key elements driving this expected growth are an increase in the incidence of urticaria, growing treatment options including the introduction of biologic treatment modalities.
Also, North America is expected to account for 41% of market growth through 2026, and the region’s growth rate is forecast to be faster than overall market growth.
Major competitive vendors that provide or are developing related treatments include:
-
Bruton
-
Celldex
-
Allakos (ALLK)
-
Amneal Pharmaceuticals (AMRX)
-
Bayer AG (OTCPK:BAYZF)
-
F. Hoffmann La Roche
-
GlaxoSmithKline Plc (OTCPK:GLAXF)
-
Johnson & Johnson (JNJ)
-
TerSera Therapeutics
-
Teva Pharmaceutical Industries (TEVA)
-
United BioPharma
-
Viatris (VTRS)
The company is also pursuing treatment conditions in other major markets.
Third Harmonic Bio Financial Status
The firm’s recent financial results are typical for a clinical-stage biopharma in that they feature no revenue and significant R&D and G&A costs associated with its pipeline development efforts.
Below are the company’s financial results for the past two and 1/2 years:
Company Statement Of Operations (SEC EDGAR)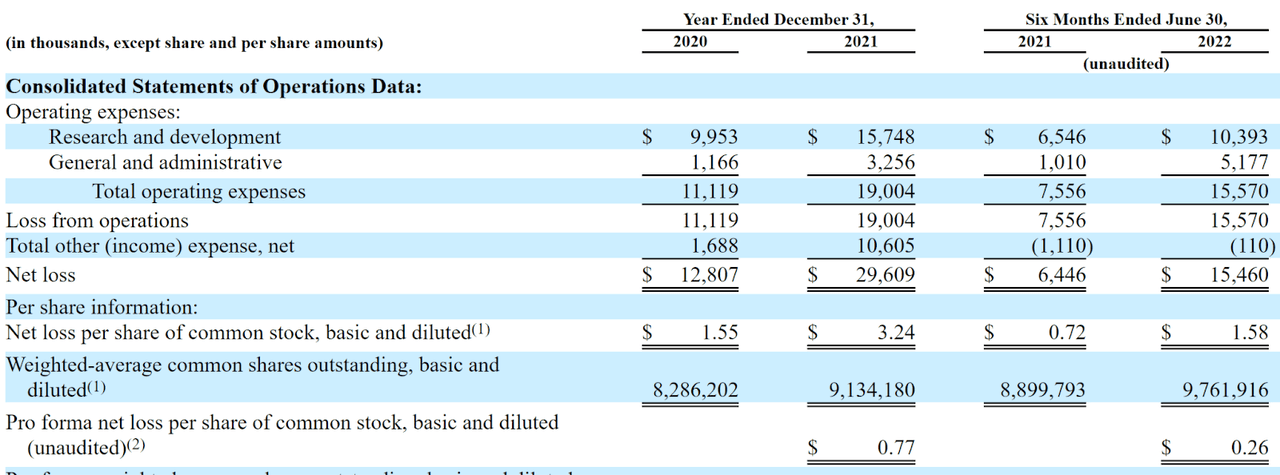
As of June 30, 2022, the company had $128.3 million in cash and $5.7 million in total liabilities.
Third Harmonic Bio IPO Details
Third Harmonic intends to raise $150 million in gross proceeds from an IPO of its common stock, although the final figure may differ.
No existing shareholders have indicated an interest to purchase shares at the IPO price, although this element may become a feature of the IPO if disclosed in a future filing.
Management says it will use the net proceeds from the IPO as follows:
to advance the continued clinical development of THB001 for the treatment of urticaria, including in a Phase 1b clinical trial for chronic inducible urticaria and in a Phase 2 clinical trial for chronic spontaneous urticaria;
to advance the continued clinical development of THB001 in additional indications, including a Phase 1b clinical trial for asthma and to fund further development or acquisition of future programs to advance through nonclinical and clinical development; and
the remainder for potential expansion of our pipeline and other research and development activities, as well as for working capital and other general corporate purposes.
(Source – SEC)
Management’s presentation of the company roadshow is not available.
Regarding outstanding legal proceedings, management said the firm is not currently a party to any proceedings that would have a material adverse effect on its financial condition or operations.
Listed bookrunners of the IPO are Morgan Stanley, Jefferies, Cowen, and LifeSci Capital.
Commentary About Third Harmonic’s IPO
THRD is seeking U.S. capital market investment to fund further trials for its product pipeline.
The firm’s lead candidate, THB001, is in Phase 1 trials for various dermatologic and respiratory condition treatments including for chronic hives and asthma.
THB001 intellectual property was in-licensed from Novartis Pharma and the firm is allowed under its agreement with Novartis to utilize third party intellectual property to further develop THB001.
THRD management is exploring other development opportunities for its THB001 as a treatment for mast cell-caused conditions.
The market opportunities for treatments for these conditions are large and expected to grow at moderate rates of growth over the coming years.
The company’s investor syndicate includes major pharma firm Novartis among several well-known life science venture capital firms.
Morgan Stanley is the lead underwriter, and IPOs led by the firm over the last 12-month period have generated an average return of negative (34%) since their IPO. This is a bottom-tier performance for all major underwriters during the period.
When we learn further information about the IPO’s pricing and valuation assumptions, I’ll provide a final opinion.
Expected IPO Pricing Date: To be announced.


Be the first to comment