Morsa Images
A Quick Take On Prime Medicine
Prime Medicine (PRME) has filed to raise $100 million in an IPO of its common stock, according to an S-1 registration statement.
The firm is a pre-clinical stage biopharma developing genetic therapies for the treatment of various serious medical conditions.
I’ll provide a final opinion when we learn more IPO details from management.
Prime Overview
Cambridge, Massachusetts-based Prime Medicine was founded to develop its Prime Editing technologies in-licensed from the Broad Institute, some of which have been granted a patent, which may enable the creation of genetic editing treatments for diseases such as Sickle Cell Disease, Wilson’s Disease, and various neuromuscular diseases.
Management is headed by president and CEO Keith Gottesdiener, M.D., who has been with the firm since July 2020 and was previously CEO of Rhythm Pharmaceuticals after leading Merck’s late clinical development organization.
The firm’s lead program, which is still in discovery phase, is seeking to treat Sickle Cell Disease.
It is one of currently 18 programs, of which 17 are being developed solely in-house and the 18th, its Sickle Cell Disease, is being developed in partnership with Beam Therapeutics.
Below is the current status of the company’s drug development pipeline:
Company Pipeline Status (SEC EDGAR)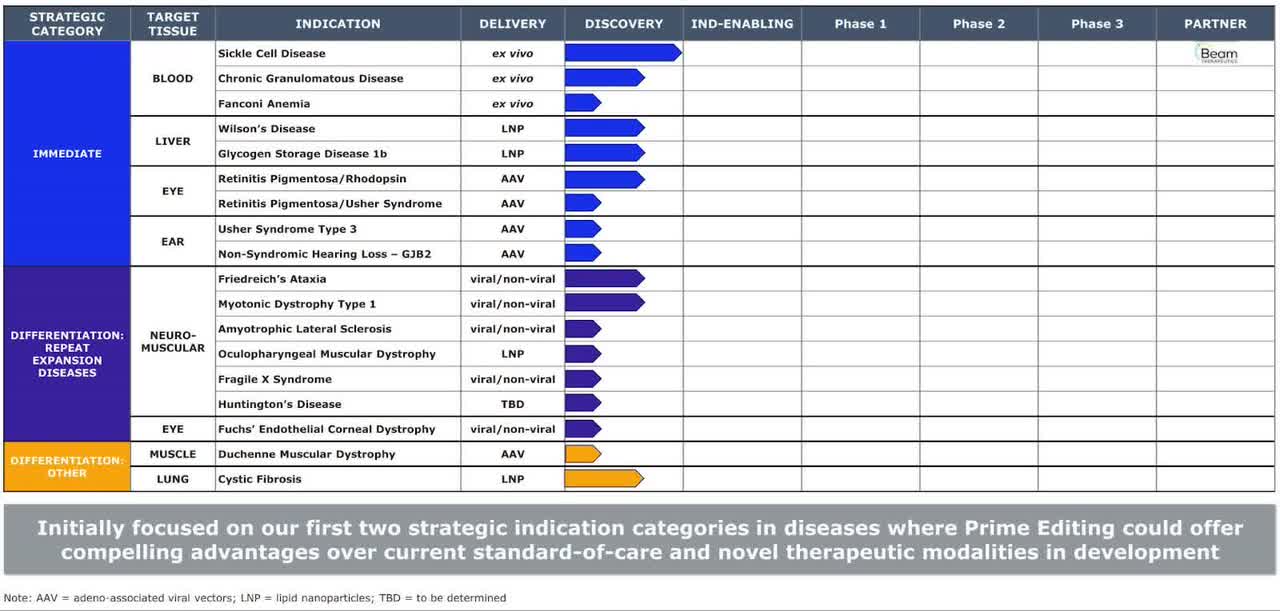
Prime has booked fair market value investment of $425.5 million as of June 30, 2022 from investors including GV (f.k.a. Google Ventures), ARCH Venture Partners, F-Prime Capital Partners and Newpath Partners.
Prime’s Market & Competition
According to a 2018 market research report by Grand View Research, the market for sickle cell disease treatment is expected to reach $5.5 billion by 2023.
This represents a forecast CAGR (Compound Annual Growth Rate) of 14.3% from 2019 to 2023.
Key elements driving this expected growth are a growing patient pool size leading to increased demand and development of potential new drugs in late stages, including voxelotor, crizanlizumab, Altemia and rivipansel.
Also, currently available treatments for sickle cell disease typically provide only symptomatic relief and palliative care, such as blood transfusions, pharmacotherapy and bone marrow transplants.
Major competitive vendors that provide or are developing related treatments include:
-
bluebird bio
-
CRISPR Therapeutics
-
Editas Medicine
-
Intellia Therapeutics
-
Sangamo Therapeutics
-
AVROBio
-
Freeline Therapeutics
-
Others
Prime Medicine Financial Status
The firm’s recent financial results are typical for a development stage biopharma in that it has received no revenue (except for some collaboration revenue in 2020) and has had significant R&D and G&A expenses associated with its discovery efforts.
Below are the company’s financial results for the past two and 1/2 years:
Company Statement Of Operations (SEC EDGAR)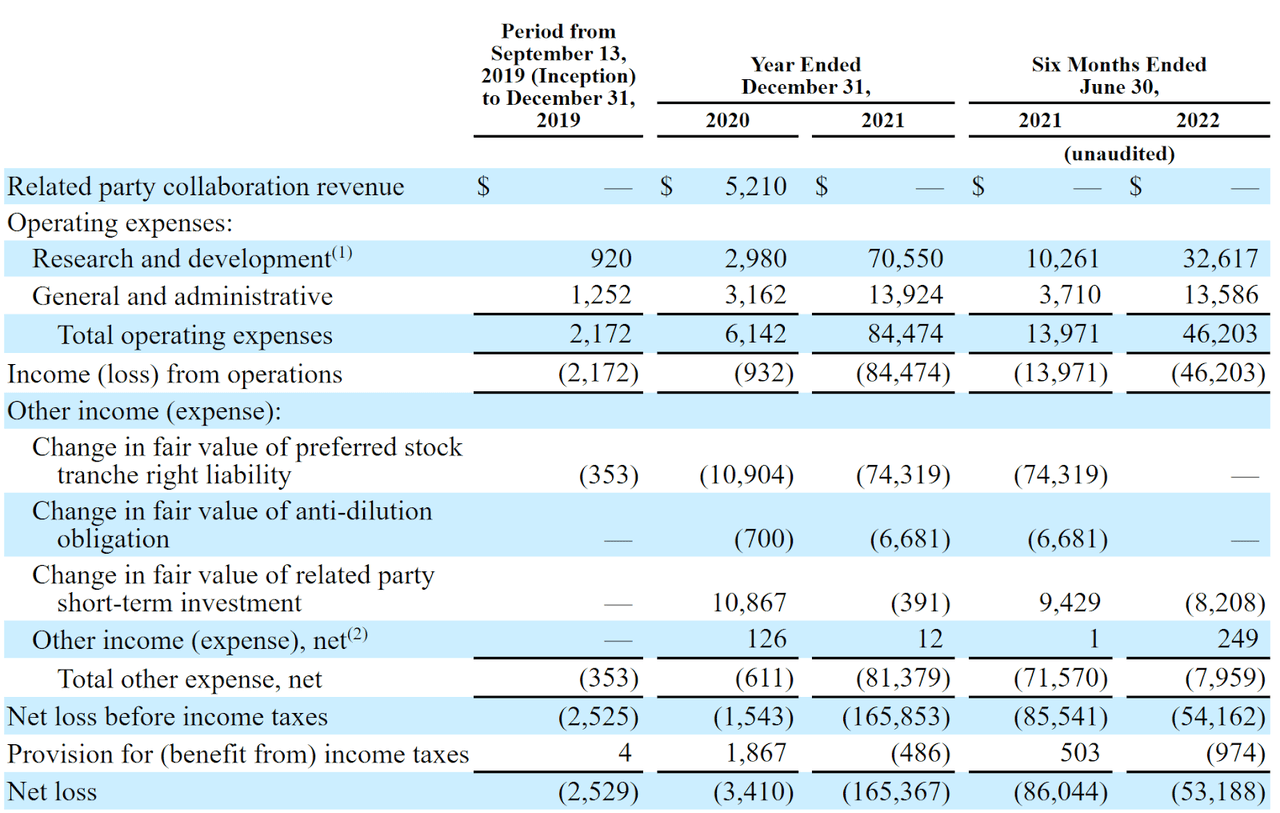
As of June 30, 2022, the company had $92.2 million in cash and $46.9 million in total liabilities.
Prime Medicine’s IPO Details
Prime intends to raise $100 million in gross proceeds from an IPO of its common stock, although the final figure may be higher.
N existing shareholders have indicated an interest to purchase shares at the IPO price, although this element may become a feature of the IPO if disclosed in a future filing.
Management says it will use the net proceeds from the IPO as follows:
for continued research and development of our immediate target indications and differentiation target indications, including through achieving preclinical proof-of-concept in several programs;
to develop our early-stage manufacturing processes and build out our dedicated chemistry facility; and
the remainder for general corporate purposes.
(Source – SEC)
Management’s presentation of the company roadshow is not available.
Regarding outstanding legal proceedings, management said the firm is not a party to any litigation but also cautioned that the legal environment surrounding gene editing technologies is ‘highly dynamic’.
Listed bookrunners of the IPO are JPMorgan, Goldman Sachs, Morgan Stanley and Jefferies.
Commentary About Prime’s IPO
PRME is seeking substantial U.S. public capital market investment to advance its large pipeline of genetic treatment candidates into clinical trials.
The firm’s lead candidate, which is still in discovery phase, is seeking to treat Sickle Cell Disease.
The market opportunity for the various programs management seeks to treat are large in the aggregate and are expected to grow at varying rates.
Management has disclosed a collaboration with Beam Therapeutics (BEAM), a genetic treatment company.
The company’s investor syndicate includes well-known and highly regarded life science venture capital firms.
JPMorgan is the lead underwriter and IPOs led by the firm over the last 12-month period have generated an average return of negative (36.1%) since their IPO. This is a lower-tier performance for all major underwriters during the period.
Gene editing therapy approaches are becoming more common but face long US FDA approval processes as they are still very new, very powerful and still not widely tested.
Additionally, these approaches can be extremely expensive, so reimbursement of genetic therapies can be challenging, as some therapies have costs of up to $2.8 million per treatment.
When we learn more about the IPO, I’ll provide an update.
Expected IPO Pricing Date: To be announced.


Be the first to comment