24K-Production
The most challenging-to-treat mental health disorders, including PTSD, major depressive disorders, addictions to alcohol and drugs, anxiety, and eating disorders, lead to the deaths of thousands of people each year and to untold suffering for the patients and their families.
Many patients do not respond to current treatments, and no pharmacological therapy is available for many serious mental illnesses.
COMPASS Pathways plc (NASDAQ:CMPS) is a UK-based company trying to use the Psychedelic drug Psilocybin to treat some of the most challenging mental illnesses. I will present my view that:
- 1. The drug has an unusually high chance of passing its upcoming phase III trial.
- 2. The regulatory infrastructure in the U.S. is likely to approve the drug within two years.
- 3. If approved, the drug will be the first entrant into a multi-trillion dollar total addressable market (“TAM”).
Getting Approved
To be approved, a drug must be safe to use and effective. That is the purpose of a medical trial.
Psychedelic compounds have been used by humans for many years (in some cases, centuries), sometimes as part of religious ceremonies but often for their hallucinogenic properties. Psilocybin is the active ingredient in Magic Mushrooms, extensively used in the past.
As a result of this usage we already know two important facts:
- They are well tolerated by Humans.
- They affect the way the brain works.
Many companies are trying to develop treatments using a variety of psychedelic compounds. I will be working through them and writing about them in the coming months, looking for more to invest in and for those to avoid. In this article, I will be concentrating on COMPASS Pathways.
I started my research by examining what medical trials are taking place now and built the following table.
Ongoing trials in the field (Author generated)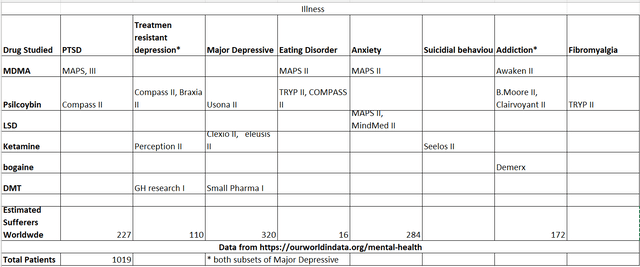
The table is not exhaustive; however, it contains the companies I could find currently running a trial and the drug tested. (Trials of psychedelics in other areas, e.g., severe pain, are also ongoing put not on the table.)
The compound with the most trials in place is Psilocybin 9 phase two trials looking at six different mental illnesses. Those six various illnesses represent more than a 350 million patient population. CMPS is running three of these trials. Having decided to look at CMPS, I investigated evidence around its candidate drug COMP360, a proprietary Psilocybin compound.
Does Psilocybin Work?
A search of the National Library of Medicine delivered 22 hits for Meta-Analysis Psilocybin. (meta-analysis is a research article looking at multiple trials) The latest was from February 2022 and looked at single or multi-dose trials of Psilocybin. It looked at ten studies and reported:-
The present study demonstrates that single- or two-dose psilocybin administration has rapid and sustained antidepressant effects for up to 6 months, with favorable cardiovascular safety and acceptability.
Of the 22 Meta-Analysis, nine covered depression (three included anxiety), two looked at addiction, one behavioral disorder, five were general reports, and the rest looked at side effects.
All 22 studies were positive for Psilocybin, showing very few significant side effects and sustained and significant improvement in all patient populations.
One general analysis considered 13 trials between 1969 and 2020, concluding that the different psychedelic drugs have different neuropsychological consequences. It also said
Psychedelic compounds have shown the ability to increase neuroplasticity. They offer a chance to alter brain activity, reduce the suffering of victims, and perhaps ultimately a cure to many mental illnesses for a large percentage of sufferers.
My study of published meta-analysis suggests that Psilocybin has a pronounced and durable effect on many mental illnesses and has few severe side effects. The problem is that the available studies are not of the standard that the FDA will need to authorize these compounds for use.
CMPS Psilocybin drug trials.
Last month, COMPASS Pathways released the results of the highly anticipated phase II trial of its Psilocybin assisted therapy.
The FDA granted COMPASS Pathways Breakthrough Therapy Designation (BTD) in October 2018. It was an important step and means the FDA has been working with CMPS to accelerate the drug’s approval process. The BTD followed the 2015 Phase I study when compass dosed 19 patients with its Psilcoybin compound and found promising signals in treatment-resistant depression. A fuller explanation is in this article quoted below
The FDA designates a drug as a Breakthrough Therapy if preliminary clinical evidence shows that it may demonstrate substantial improvement over available therapy. Breakthrough Therapies are supported by the FDA throughout the clinical development programme to ensure as efficient a process as possible.
In the Phase 2 trial, designed to work out the correct dosage for larger stage 3 trials, CMPS tested its psilocybin formula COMP360 for patients with treatment-resistant depression (TRD). TRD patients have tried at least two other forms of treatment without benefit and are considered the hardest subset of major depressive illness patients to treat.
Market reaction to Phase II results
The market did not receive the trial results well, and the stock price fell. It is my view that the market completely misinterpreted these results. Given that the patient group had already tried two forms of treatment, 25% of them meeting the primary endpoint of a sustained response on the (MADRS) scale at 12 weeks is a very positive outcome. It did mean that 75% of patients did not respond, which shows the drug will not be a panacea for eliminating depression, and we do not have data on the durability beyond 12 weeks from this study. However, the results are better than any other treatment for this group of patients.
Safety, this again was misinterpreted in my view; five patients reported suicidal ideation or behavior and self-injury. Compass CEO said the five patients were in the non-responder group; The Pollan effect may be the case here. Bearing in mind the nature of the group, this may well explain this negative impact, and COMP360 may not be a cause at all. Patients in the study may have raised their hopes so much that they may have become desperate when the drug did not help them.
In April this year, a further study was presented; twelve patients who had previously tried at least five anti-depressant treatments were given a single 25mg dose with therapy support. 58% of patients showed the required MADRS response at 12 weeks and reported no adverse events or suicidal tendencies.
CMPS expects to begin the phase III trial in Q3 2022; the protocols have been submitted to the FDA before launching this large-scale multi-center international trial. The results will be pivotal for CMPS, the FDA will approve the drug, or not, when they receive the results.
Anorexia Nervosa
The preoccupation with weight and shape is one of the most challenging disorders to treat and currently has no pharmacological treatment. In April, COMPASS presented the results of an investigator lead study into Anorexia Nervosa. This serious mental illness carries the highest mortality rate of psychiatric disorders, with up to 40% of deaths coming from suicide. In the trial, 40% of patients experienced clinically significant reductions in eating disorder psychopathology at the 3-month follow-up, and 50% demonstrated statistically significant decreases in eating concerns. 90% of patients thought the treatment had helped them, and no adverse reactions were recorded.
COMPASS announced in July 2022 that they would be pressing on with a double-blind, randomized controlled phase II trial of COMP360 in anorexia nervosa. The trial will be of FDA standard and take place in the UK and the US with 60 patients.
COMPASS has also initiated a Phase II trial looking at COMP360 with patients who have PTSD.
How can one drug be expected to treat all of these different mental disorders?
The brain’s capacity to change, its neuroplasticity, makes a cure possible. This video explains that Psilocybin affects the serotonin system in the brain, a widespread complex system of receptors of which one, Serotonin 2A, involved in regulating mood, sleep, and thinking process, is affected by Psilocybin. The compound increases the brain’s neuroplasticity, allowing different brain regions to connect in new ways; connections causing unhealthy states such as depression and addiction can be reset healthily.
The drug allows the brain to reset unhealthy patterns and, as a result, may work on all unhealthy patterns. Which explains the positive effects found in every study in every area investigated.
Treatment consists of the drug and therapy both before and after to try to rest the new healthy brain patterns
The financials
The average price target from the nine analysts following COMPASS pathways is $68 against a current price of $14.9, implying a 356% upside and a target market cap of $3 billion. I value COMPASS pathways at a much higher figure.
Suppose COMPASS can get its drug approved for treatment-resistant depression. In that case, the following is a reasonable forecast. (the price of $1,000 per patient is my estimate). COMPASS has not indicated the cost. I used figures from a meta-analysis of MDMA to get a ballpark idea:
Results: MDMA-AT as conducted in the phase 3 trial costs $11,537 per patient.
TRD potential, patients from earlier table (Author)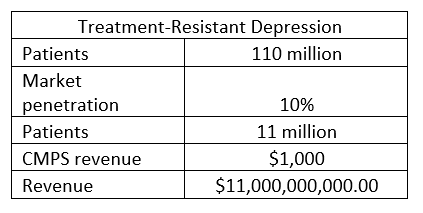
The largest pharmaceutical in 2021 was Johnson and Johnson, with a turnover of $93 billion; Johnson and Johnson has a market cap of $449 billion. So the market cap is 4.8 x Revenue.
Using 4.8 as a multiplier, COMPASS pathways would have a market cap of $52.3 trillion. (I am not suggesting this as a target, just showing the potential)
Anorexia market potential (Author)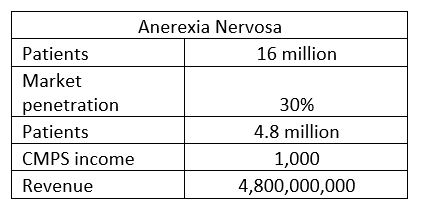
The 4.8 multiplier gives $23 trillion.
The potential is clear, but can they get the drug approved?
The Cash Position
At the recent Q2 earnings call, after a question from the Citi analyst, CFO Mike Falvey explained that the cash burn for the quarter of $37 million included an $18 million FX charge on converting GBP denominated cash balances to USD for reporting purposes. As COMPASS have both U.S. and GBP cash balances, this $18 million was a paper loss only as they satisfy dollar payables in dollars and pound payables in pounds. Adjusting, we get a cash burn of $19 million; they ended the period with $207 million cash on the balance sheet. That equates to about ten quarters at the current burn rate, but as they are beginning some significant trials later this year, we should probably cut that to 8 quarters or two years.
How long will it take to get the drug to market?
The Phase II trial started recruiting patients in 2019, and with results out inside two years, the phase III trial may follow a similar time frame. The Breakthrough Therapy Designation and the large unmet need should ensure some urgency behind this trial. If overwhelming efficacy is found during the trial (the reported investigations suggest this is likely), then the oversight board will recommend ending the trial early. For this to happen, the trial would have to meet its endpoints statistically significantly and meet any prespecified stopping guidelines. We will have to wait until October 12th to find the trial terms.
The U.S. administration appears to be preparing to authorize the use of Psychedelic therapies within the next two years, tying in with this timeline. In a letter dated May 13th 2022, from the SAMHSA (secretary for mental health and substance abuse) to Madeleine Dean at the House of Representatives, the following two quotes can be seen.
…anticipated approval by the Food and Drug Administration (FDA) of 3,4-methylenedioxymethamphetamine (MDMA) for the treatment of Post-Traumatic Stress Disorder and Psilocybin for the treatment of depression within approximately 24 months.
….SAMHSA agrees that too many Americans are suffering from mental health and substance use issues, which have been exacerbated by the ongoing COVID-19 pandemic, and that we must explore the potential of psychedelic-assisted therapies to address this crisis
Conclusion
If COMPASS pathways gets its COMP360 drug approved the potential market is huge, trillions of dollars. The drug is going to a phase III trial this year and, based on the evidence of previous trials, has a good chance of success.
The U.S. administration appears to be getting ready to authorize the drug and the FDA is working with CMPS under the Breakthrough Therapy Designation due to the perceived high chance of success and the unmet need amongst a large patient population.
It seems a compelling investment thesis, and I bought CMPS @ $16.11. I have also bought Atai Life Sciences (ATAI), a substantial investor in CMPS, at @ $4.13 and will be writing about them shortly.


Be the first to comment