
designer491
On Monday Nov 14, Stoke Therapeutics (NASDAQ:STOK) reported interim data from their p1/2 trials in children and adolescents with Dravet Syndrome, a severe and progressive genetic epilepsy.
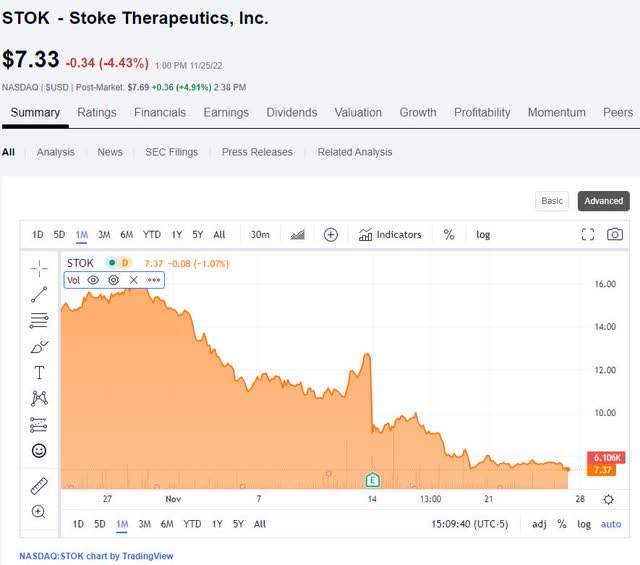
Looking at the recent 1-month chart (see below), it’s easy to think that the interim data did nothing to reverse, or perhaps even contributed to, the stock’s steady decline.
Seeking Alpha
Since Nov 14, the stock has declined 41%, from $12.49 (on Nov 11) to $7.33 (on Nov 25). Or year-to-date, the stock is down by 68%, from $23.01 (on Jan 3) to $7.33 currently.
The chart is certainly not a pretty sight. However, in my mostly small cap, clinical-stage biotech portfolio, 11 out of 18 positions have a >60% YTD decline, and most don’t have company-specific reasons to account for the decline.
All this is to say that in my opinion, the current bear market condition may be more responsible for the significant decline in so many small cap, clinical-stage biotech stocks, than any company-specific news.
Bearing this view in mind, let us now turn to STOK’s approach.
Stoking protein production with ASO (antisense oligonucleotide) drugs
According to a 2020 review, there are at least 9 FDA-approved ASO drugs treating various orphan diseases, e.g. Biogen’s (BIIB) Nurinersen for SMA, Alnylam’s (ALNY) Patisiran & Ionis’ (IONS) Inotersen for hATTR and Sarepta’s (SRPT) Eteplirsen, Golodirsen, Casimersen for DMD etc.
The same review also lists some 39 ASO candidates in ongoing development for various indications.
Suffice to say that ASO drugs have already shown clinical/regulatory successes (by targeting disease cause at a RNA level, successfully demonstrating efficacy in clinical trials and being approved/launched).
In simple terms, ASO drugs are able to bind to specific RNA sequence of interest, which results in regulating a disease-specific protein’s production. Depending on the disease, this ASO drug-affected regulation can be to turn up (increase) or to turn down (decrease) protein production.
In the case of STOK, they seek to treat diseases that have only one copy of a gene functioning normally, while the other copy is mutated, or “haploinsufficiencies“. Therefore, they design their ASO drugs to “stoke” (increase) the protein production in the healthy copy of the gene to make up for the insufficient protein production by the mutated, dysfunctional copy.
In Dravet Syndrome, the target gene is the SCN1A gene and the target protein is NaV1.1 protein. By restoring the levels of NaV1.1 protein, STOK believes that STK-001 has the potential to be the first disease-modifying treatment for Dravet syndrome.
Let us now turn to the interim data.
Interim data from p1/2a trials of STK-001 in children and adolescents with Dravet syndrome
Currently, the company has two ongoing phase 1/2a trials, namely MONARCH in the US, and ADMIRAL in the UK; as well as two follow-on extension studies, SWOLLOWTAIL in the US, and LONGWING in the UK.
The reported data is pooled data mainly from MONARCH and ADMIRAL.
The interim data is summarized below:
| Key Safety data |
|
“The interim safety analysis reported today was based on data from 55 patients who were treated with single or multiple doses of STK-001 (10mg, 20mg, 30mg, 45mg) and followed for up to six months after their last dose.
|
| Key Efficacy data |
|
“The interim efficacy analysis was based on data from 27 patients who were treated with multiple doses (20mg, 30mg, 45mg) and followed for three months after their last dose.
|
| Key PK and CSF exposure findings |
|
(Source; emphasis added)
For more details, please see this presentation on STK-001 interim analysis and listen to the conference call.
Below, I note more items, that were further covered in the conference call, which I find important.
|
1. |
The 55 patients, who were treat with one or more doses of STK-001, have “severe disease” and “are refractory to standard treatments”, which is evident in the fact that 78.1% (43/55) of them have 3 or more concomitant anti-seizure medications or 50.9% (28/55) of them are taking 4 or more Also, 50.9% are taking Fenfluramine. My takeaway: Efficacy signal (seizure reduction) observed in STOK’s p1/2a trials (n=27) is additional to other treatments’ effect. |
| 2. |
“33% (18/55) of patients experienced CSF protein elevation (>50mg/dL) after dosing; no clinical manifestations were observed” (slide 13) In response to a question on this, Dr Ticho, the CMO, said that 10% of patients are found to have elevated CSF protein at the baseline, and they are monitoring this closely. My takeaway: According to STOK, the elevated CSF protein is a known phenomenon and is being monitoring closely [not a safety concern]. |
| 3. |
“Early Indication of Improvements in Non-Seizure Comorbidities as Measured by BRIEF-P* (slide 20) My takeaway: Unlike other anti-seizure [only] drugs, STK-001 has the potential to improve the non-seizure comorbidities also, due to its upstream, root cause-targeting mechanism of action. |
(complied by author from Company Presentation)
In summary: From these positive, admittedly early data (on safety, efficacy & PK, CSF exposure), the company believes that they are on track to identify the optimal dose & dosing frequency for the phase 3 trial.
They plan to report more data in 2023 from the ongoing p1/2a and extension studies.
Investment Consideration
During the conference call, it was said that most patients are on multiple anti-seizure drugs and some are on as many as 7 different drugs. Still the seizure in these heavily-medicated patients is far from being well-controlled.
From this, one can easily conclude that Dravet syndrome continues to be a serous, yet-to-be-met medical need, in spite of having at least two specifically approved treatments in recent years, e.g. Zogenix’s fenfluramine, GW Pharmaceutical’s Epidiolex.
Besides STK-001, there are at least two other potential treatments: Eisai’s Lorcaserin (p3 topline expected Q2 2023); Longboard Pharma’s (LBPH) LP352 (p1b/2a basket study data expected in H2 2023).
Lorcaserin is an “approved” weight-loss drug, but in 2020 the FDA requested its voluntary withdrawal due to increased occurrences of cancer in patients. This FDA’s request was based on the data from a large post-approval, placebo-controlled study (n=12000, over 5 years). Eisai, Lorcaserin’s manufacturer, complied.
From the original approval label, I notice that the dose and treatment duration for weight loss in adults (10mg twice daily for 12 weeks or longer) is similar to what’s being studied in Dravet syndrome children (n=58; 2 years or older), i.e. 5mg, 10mg, 20mg per day for 14 weeks of core treatment, plus extension.
With the aforementioned cancer risk that stopped the use of Lorcaserin as a weight loss drug in adults, I’m unsure how Lorcaserin can ever be approved for any other indication with similar dose/treatment duration, even if a new [much smaller, 58 vs. 12000] placebo-controlled trial shows efficacy.
Or even if it does gets approved, will the physicians overlook the clearly-established cancer risk and prescribe to patients anyway?
Regardless, Dravet syndrome is a serious unmet need that has a sizable market.
For example, Jazz Pharmaceuticals which bought GW Pharma reported a $196.2M sale of Epidiolex in Q3 2022, a 22% increase from Q3 2021.
It’s worth noting that these treatments, existing or potential, are not directly competing for market share, as they are being prescribed as concomitant treatments, once approved.
As for estimating the potential upside, if/when STK-001 is successfully developed/approved, the examples of other successful ASO drug developers may be useful (see below).
|
ASO drug developers (stage) |
Market Cap (Nov 25, 2022) |
| IONS (commercial) | $5.9 B |
| ALNY (commercial) | $26.17 B |
| SRPT(commercial) | $10.06 B |
| STOK (clinical) | $289.03 M |
(complied by author from SA info)
One last thing: I note that both Dr Kaye, the CEO and Dr Ticho, the CMO are experienced veterans in ASO or RNA treatment space, with Dr Kaye being the former CEO of SRPT and Dr Ticho has worked in various R&D leadership roles in MRNA, PFE, and BIIB.
Financials
As of September 30, 2022, STOK has a cash runway of $252.2 M and a net loss in Q3 2022 is $26.1M. The company expects to be able to fund the operation into 2025.
Concluding Thoughts
In October 2022, the U.S. FDA has granted Rare Pediatric Disease Designation to STK-001 for the potential treatment of patients with Dravet syndrome.
Besides STK-001, the company has another program, i.e. autosomal dominant optic atrophy (ADOA), for which STOK has started a natural history study, since Aug 2022.
Also a genetic disease caused by haploinsufficiency, ADOA is said to be “the most common inherited optic nerve disorder seen in clinical practice” and up to 46% of ADOA patients are registered as legally blind. Currently, there is no approved treatment for ADOA.
For anyone who is interested in this company, please remember that any drug development is a lengthy, difficult, costly process with no guaranteed of success. Therefore, having a suitable time frame, risk tolerance and sizing is essential to be a happy & hopefully profitable investor, in my opinion.
As I mention in the start, the current macro market condition seems to be playing a big role in the decline of many clinical-stage biotech stocks indiscriminately. I don’t know if or when this [bear market condition] will change for the better. So perhaps there’s no urgency in buying for any who decide to.
As the saying goes: it’s not timing the market, it’s time in the market that matters, meaning it’s more important that one invests, than when one invests.
I agree whole-heartedly with this view.
Company-specific risks include but no limited to: delays and/or failures in trials and/or regulatory process in Dravet syndrome, ADOA or other future programs, dilution risks, delay and /or failures in commercialization of any approved drugs.
Thanks for reading. All the best!


Be the first to comment