DanaHenryPhotography/iStock via Getty Images
In the most important moment for the company, Anavex (NASDAQ:AVXL) will be presenting results from its phase 2b/3 trial on blarcamesine/Anavex 2-73 at the Clinical Trials on Alzheimer’s Disease conference in San Francisco on December 1st. While it is impossible to tell exactly what these results will be, previous trial results – albeit with a small number of participants – give some clues.
The first and perhaps most important clue is that blarcamesine is likely a disease-modifying treatment for at least some patients with mild Alzheimer’s disease:
Disease Modifying (Taiwanese Journal of Psychiatry) Anavex 2-73 at 148 weeks (Anavex Corporate Presentation)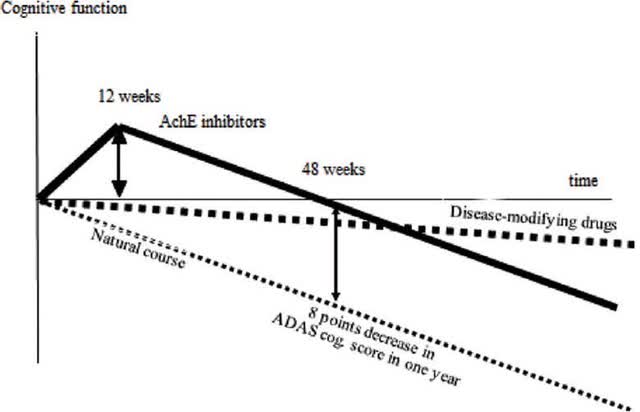
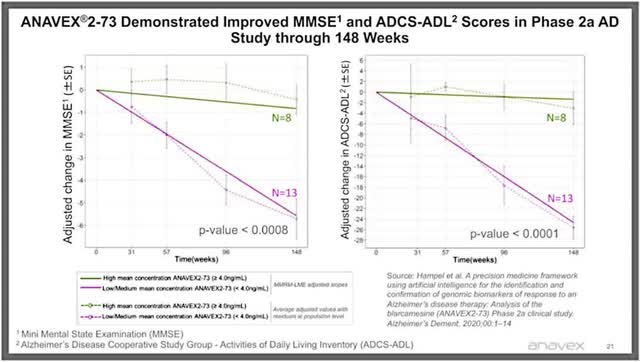
(Note the slope is more important than the specific tests).
Both blarcamesine/Anavex 2-73 and Aricept (an acetylcholinesterase enzyme inhibitor or AchE) are sigma-1 receptor agonists which inhibit the release of intracellular calcium and subsequent oxidative stress. Both may be direct antioxidants. It is possible that blarcamesine is a better sigma-1 receptor agonist and/or better antioxidant than Aricept and that is why in some individuals with mild Alzheimer’s disease it can nearly stabilize the disease for almost three years at high concentrations. For moderate Alzheimer’s disease, blarcamesine is much less effective because intracellular calcium release decreases after the early stages of the disease (although oxidative stress remains a problem as the result of the influx of calcium) (figure 3).
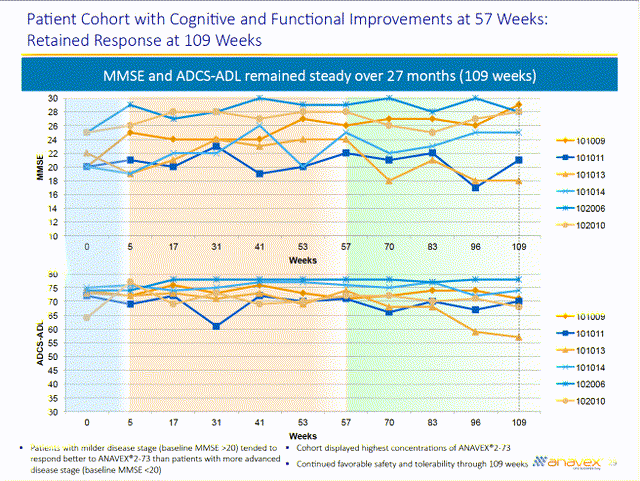
The second clue as to the effectiveness of blarcamesine for Alzheimer’s disease comes from 57 week and 109 week results. Six individuals with mild Alzheimer’s disease (sometimes defined as an MMSE of 20 to 24) or mild cognitive impairment showed improvements at 57 weeks. Five out of six of these individuals retained these improvements at 109 weeks (one at a high concentration declined).
4 out of 6 patients who saw improvements at 57 weeks were at high concentrations (no one in the mid-concentration group improved). The breakdown is as follows:
Two out of nine patients at low concentrations improved (although the improvement in the first patient requires some explanation):
Baseline 57 weeks 109 weeks
1009 20 26 29
1011 20 22 21
Three out of nine patients at high concentrations improved
1014 20 25 25
2006 25 29 28
2010 25 28 28
About two-thirds of those at high concentrations had mild Alzheimer’s disease or mild cognitive impairment, so 3 out of 6 individuals in this group improved over the course of 109 weeks. On the other hand, no one with moderate Alzheimer’s disease improved on blarcamesine.
To a certain extent the effectiveness of blarcamesine is reduced by genetic mutations that increase intracellular calcium release and oxidative stress in mild Alzheimer’s disease, namely mutations in sigma-1 receptor and COMT (Catechol-O-methyltransferase) genes.
Effect size on clinical outcomes at week 5
|
MMSE |
ADCS‐ADL |
|||||
|
Subjects’ characteristics |
Mean Δ at 57 weeks |
Cohen’s d |
N (%) |
Mean Δ at 57 weeks |
Cohen’s d |
N (%) |
|
All |
−1.52 ± 4.15 |
0.57 a |
21 (100.0%) |
−5.24 ± 8.42 |
0.18 |
21 (100.0%) |
|
Baseline MMSE ≥ 20 |
−0.15 ± 4.06 |
0.94 b |
13 (61.9%) |
−2.08 ± 5.88 |
0.66 a |
13 (61.9%) |
|
Absence of SIGMAR1 p.Gln2Pro variant |
−0.62 ± 4.11 |
0.81 b |
16 (76.2%) |
−3.38 ± 7.60 |
0.43 |
16 (76.2%) |
|
Absence of COMT p.Leu146fs variant |
−0.62 ± 4.05 |
0.81 b |
16 (76.2%) |
−2.44 ± 6.93 |
0.57 a |
16 (76.2%) |
|
Absence of SIGMAR1 p.Gln2Pro variant and baseline MMSE ≥ 20 |
0.27 ± 4.29 |
1.01 b |
11 (52.4%) |
−1.36 ± 6.09 |
0.76 a |
11 (52.4%) |
|
Absence of COMT p.Leu146fs variant and baseline MMSE ≥ 20 |
0.18 ± 4.35 |
0.98 b |
11 (52.4%) |
−0.73 ± 5.29 |
0.89 b |
11 (52.4%) |
|
Absence of SIGMAR1 p.Gln2Pro variant and absence of COMT p.Leu146fs variant and baseline MMSE ≥ 20 |
0.50 ± 4.45 |
1.05 b |
10 (47.6%) |
−0.40 ± 5.46 |
0.93 b |
10 (47.6%) |
(Table 3)
An MMSE score of 20 or above, though, has a greater impact on the effectiveness of blarcamesine than the absence of variants.
Anavex has now limited its phase 2b/3 trial to those with mild Alzheimer’s disease and mild cognitive impairment (i.e. equal to or greater than 20), so the percentage of those with mild Alzheimer’s disease or mild cognitive impairment who improve on the highest dose in the current phase 2b/3 clinical trial at 48 weeks could be over sixty percent (four out of six in this category improved in the 57 week trial).
The results are more critical than any other concerns one may have about Anavex’s trial. The relatively small size of Anavex’s trial (500 participants) and that they had no U.S. sites will probably not be of concern for the FDA. The FDA wants a minimum of 300 participants in a phase 3 clinical trial and it will accept under specified conditions phase 3 trial results from other countries. If then blarcamesine meets both efficacy and safety requirements, the FDA would likely grant it at least accelerated approval (approval for sale while additional trials are conducted). A possible request for an additional 18 month trial to establish whether blarcamesine is indeed a disease modifying drug does not seem unreasonable.
So far at least, Anavex’s stock value has not increased very much based on the announcement and anticipation of its CTAD presentation. This may reflect the considerable amount of doubt raised about very small participant numbers and about the data itself. The stock may tick up a bit more as the date draws nearer. I believe the results in this much larger trail will be good, though, and that some form of FDA approval will be forthcoming. Based on that, I rate Anavex as a buy.


Be the first to comment