narvikk/E+ via Getty Images
Alpha particles are known for their high potential to directly damage the cell DNA causing tumor cell death. However, these particles cannot travel far in the tissue precluding their therapeutic use. Alpha Tau Medical Ltd. (NASDAQ:DRTS) was incorporated in 2016 in Israel to develop and commercialize a novel alpha radiation technology invented by Tel Aviv University professors Itzhak Kelson and Yona Keisari. The Company’s proprietary Alpha DaRT (diffusing alpha-emitters radiation therapy) was developed to facilitate local delivery of alpha particles, leveraging the innate decay chain of Radium-224. Small, precise amounts of radioactive Radium-224 are affixed to stainless steel sources, which are inserted into the tumor itself. Radium-224 with a half-life of approximately 3.7 days, decays to Pb-212 with a further half-life of approximately 12 hours, which eventually stabilizes to the inert Pb-208. The daughter atoms in the decay chain release cytotoxic alpha particle payloads that diffuse inside the tumor, emitting short-range alpha radiation that damages and kills cancer cells within a short span of time.
The Alpha DaRT has the potential to deliver a high dose of radiation in a small, <5 mm range, with more affinity to tumor tissue than healthy tissue, as observed in animal studies. A series of Alpha DaRTs each injected a few millimeters apart can cover an entire tumor blob, while avoiding the healthy tissue around. The US FDA granted Breakthrough Device Designation to Alpha DaRT in June 2021. Seven types of applicators have been developed including one for thin sources utilized in quick, outpatient procedures with minimal radiation exposure that do not require hospitalization.
To enable short shipping times for anticipated commercial operations, the Company is ramping up its manufacturing capabilities to ~400,000 sources per year from Jerusalem, and to ~125,000 sources per year from Lawrence, Massachusetts, and establishing a new facility in Togane, Japan.
Alpha Tau Medical’s Trials
Preclinical trials of DaRT with experimental models of mouse and human-derived tumors demonstrated a high degree of tumor ablation in cells of various histological types. It was also observed that “DaRT can be combined with chemotherapy to achieve better control of local and metastatic cancer.” The Company’s first clinical study in sites in Israel and Italy evaluated safety and feasibility in Skin / Head & Neck SCC, along with initial tumor response and local PFS (progression free survival). Results showed complete response (CR) in 78.6% and partial response in 21.4%; CR durability at ~7 months was observed in 77.2% subjects having local control, while 22.7% had local recurrence. The study also observed that the treatment was effective against radioresistant tumors where the patient median age was 80.5 years. The therapy was well tolerated with no observation of systemic toxicity, and no suppression of the immune system. Local toxicities were <= grade 2.
A pilot feasibility study in the US was conducted with 10 skin cancer patients, in 2H-2021 at 5 centers led by Memorial Sloan Kettering Cancer Center. Study results were announced in January 2022 showing 100% CRR at 12 weeks, with no product related SAEs (serious adverse events). Presently clinical trials are ongoing worldwide at leading cancer centers in various indications including skin cancer and metastases (US, Israel, Italy and Japan), oral cavity cancer (Israel, Italy and Japan), breast cancer (Russia and Japan), and pancreatic cancer (Canada). The Company is also awaiting FDA feedback on its US pivotal trial planned to be initiated in the first half of 2022.
Milestones 2022 (Company Presentation)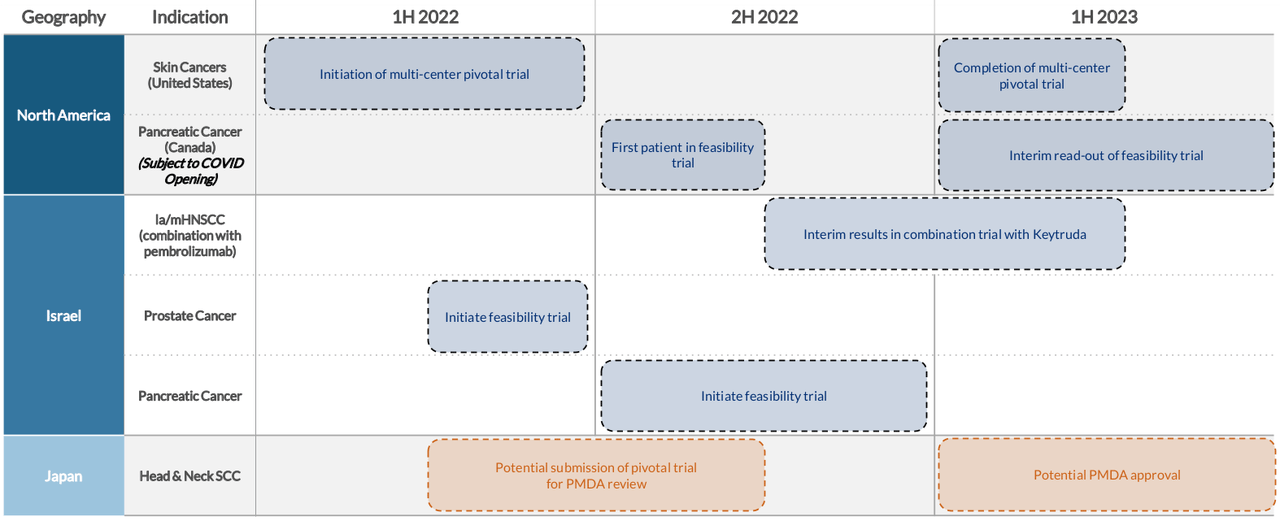
Alpha Tau’s Financials
Alpha Tau from inception through 12/31/2021, raised funds primarily through convertible preferred shares (~$57.91M), ordinary shares (~$14M) and government grants (~$5M). The Company recently consummated a business combination with SPAC Healthcare Capital Corp., infusing fresh capital. The Company anticipates that the proceeds from the business combination and the PIPE investment, together with its cash and cash equivalents of $31.93 million as of 12/31/2021, will be sufficient for a cash runway of at least 24 months. The Company presently has a market capitalization of approximately $663 million at last closing price of $9.96 on 4/10/2022, with 66.53 million shares outstanding of which 48.15% is held by the public, while insiders hold 24.99%. Private corporations and institutions hold 18.35% and 8.51% respectively. The Company’s ordinary shares and warrants commenced trading on Nasdaq on 3/8/2022 under the symbol “DRTS” and “DRTSW,” respectively. No public market existed for its ordinary shares or warrants prior to this.
Contractual obligations
The Company has various commitments of payments against intellectual property (IP) acquired. These include:
-
Royalties and additional sales proceeds for the amount of grants received from IIA plus interest at LIBOR rate, with total obligation amounting to ~$6.5 million as at 2021 year end;
-
In connection with IP from Ramot at Tel Aviv University Ltd., the technology transfer company of Tel Aviv University – 2.5% royalty on net sales of alpha radiation products and a 7% royalty on any royalties or revenues from licensing such products;
-
Grant to HekaBio K.K. options to acquire 271,588 of Alpha Tau ordinary shares at a price of $4.42 each and a royalty of 3.5% of the reimbursement price and 10% of distribution receipts in Japan if HekaBio K.K. successfully assists the Company in obtaining regulatory marketing approval of the Alpha DaRT in Japan;
-
As part of ongoing R&D activities, BGN Technologies, the technology transfer company of Ben Gurion University is to be paid a 0.75% royalty on all sales of alpha radiation products and a 1.5% royalty on sales of any products that contain IP owned by Ben Gurion University, net of certain deductions. Additionally 4% and 8% of license revenues related to IP jointly developed and IP solely developed by BGN, respectively, with IP to be owned by Alpha Tau;
-
As part of the clinical trial agreement with Cambridge University Hospitals NHS Trust, to pay 5% of any marginal increase in net sales generated on account of any patent or patent claim granted from the research performed in such trial and 2% of net sales received for the treatment of SCC of the vulva.
Risks
Alpha Tau is an “Emerging Growth Company” and a “non-accelerated filer” and a “foreign private issuer.” The Company may take advantage of exemptions from reporting requirements, including but not limited to executive compensation disclosure rules.
The Company has a limited operating history. The Company had an accumulated deficit of approximately $52.84 million as of 12/31/2021, and it will be several years before a positive cash flow is possible.
The Company’s therapeutic product is radiation technology based, which invites enhanced scrutiny by regulatory agencies. There is also risk of hostile perception and slow market acceptance by the target audience, including patients, physicians, care providers, healthcare payers and others in the medical community.
Bottomline
The Company is sufficiently funded for two years but still in an early development stage. We will wait for more data from the yet-to-be-initiated US pivotal trial and the various clinical trials before taking a call. Citi has initiated coverage with a buy rating, while shares issued pursuant to the PIPE investment come out for sale in the market.
About the TPT service
Thanks for reading. At the Total Pharma Tracker, we offer the following:-

Our Android app and website features a set of tools for DIY investors, including a work-in-progress software where you can enter any ticker and get extensive curated research material.
For investors requiring hands-on support, our in-house experts go through our tools and find the best investible stocks, complete with buy/sell strategies and alerts.
Sign up now for our free trial, request access to our tools, and find out, at no cost to you, what we can do for you.



Be the first to comment