Yoshiyoshi Hirokawa/DigitalVision via Getty Images
In June 2021, I authored the article, Aldeyra Therapeutics: A New Approach To Ocular Inflammatory Diseases With Impressive Clinical Data. I was intrigued by both the consistently strong breadth of clinical data that the company had accumulated along with the novel underlying science that promises a new approach to treating certain inflammatory diseases. Unfortunately, the past year since the publication of that article has seen a bumpy downhill ride for Aldeyra Therapeutics (NASDAQ:ALDX) shareholders and the overall meltdown in small tech biotech has exacerbated the pain. However, things are looking up again.
Background
As its lead indications, Aldeyra is pursuing ocular conditions of both dry eye disease (DED) and allergic conjunctivitis (AC). This article will be primarily focused on updating readers on the DED program which seems destined to become a significant value of shareholder value in the near term. I urge those unfamiliar with Aldeyra to start with my initial June 2021 article which explores their novel approach that targets Reactive Species Aldehydes or RASPs, molecules that are known to be associated with inflammation.
There have been several recent twists and turns related to the Aldeyra and its DED program. Some unexpected disappointments have rocked the shares while other complementary studies have produced stunningly convincing p values that mamma would be proud of. In a surprising turn of events, one key study that initially failed to hit a key primary endpoint, achieved it 5 months later when a more objective measuring tool was used to reevaluate early readings of ocular redness. This reversal reminds one of an NFL referee going to the videotape and stating, “Upon further review...” This development meant that the company had seemingly already achieved the minimum hurdle necessary for DED FDA approval.
The icing on the cake came on July 12, 2022 with the release of clinical data from the Dry Eye Disease Chamber Crossover Trial of Reproxalap, a study design whereby individual patients served as their own control in measuring multiple endpoints versus a placebo/vehicle. With patient to patient variability eliminated in a crossover design, the study achieved spectacular results that may have helped explain some earlier inconsistencies in clinical data related to patient variability.
The company now believes it has easily met its DED clinical objectives, a position that I concur with, and is preparing to submit an NDA to the FDA. A lucrative monetization event with a large pharmaceutical company for DED seems likely.
What is Reactive Aldehyde Species (RASP)?
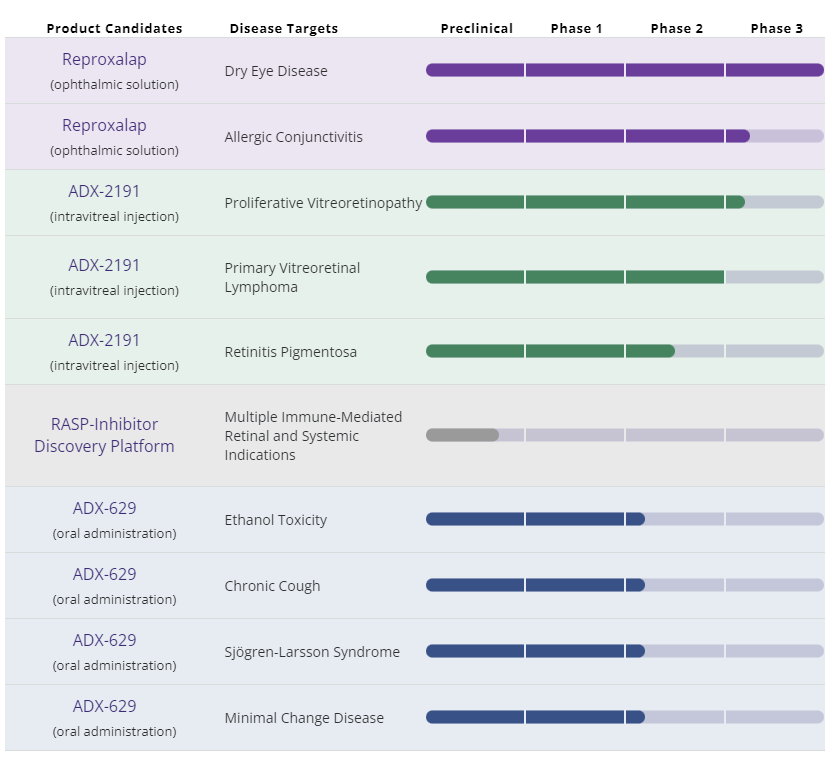
The neutralization of RASP is the underlying platform that Aldeyra’s business is based upon. In simple terms, RASP are reactive molecules in the body that sometimes bind to cellular biomolecules and disrupt their function. An over-abundance of RASP in the body can activate pro-inflammatory mediators and the result is damaging inflammation. The Aldeyra approach is to neutralize RASP, and they appear to be the only public company targeting these molecules at this time. RASP are formed by a variety of biological processes, including lipid peroxidation, alcohol oxidation, polyamine and glucose metabolism. The complete and complex definition of RASP can be found on Wikipedia. The presence of RASP in patients with DED is the basis for targeting the molecules as a cause of the underlying inflammation that is associated with DED. Reproxalap is the name of the ophthalmic solution eye drop that Aldeyra has been testing for both DED and allergic conjunctivitis. Its pipeline includes other versions of the therapy as shown in the chart below:
Aldeyra Therapeutics, Inc.
What is Dry Eye Disease?
The Mayo Clinic describes DED as follows:
Dry eye disease is a common condition that occurs when your tears aren’t able to provide adequate lubrication for your eyes. Tears can be inadequate and unstable for many reasons. For example, dry eyes may occur if you don’t produce enough tears or if you produce poor-quality tears. This tear instability leads to inflammation and damage of the eye’s surface.
Dry eyes feel uncomfortable. If you have dry eyes, your eyes may sting or burn. You may experience dry eyes in certain situations, such as on an airplane, in an air-conditioned room, while riding a bike or after looking at a computer screen for a few hours.
Treatments for dry eyes may make you more comfortable. These treatments can include lifestyle changes and eyedrops. You’ll likely need to take these measures indefinitely to control the symptoms of dry eyes.
Symptoms
Signs and symptoms, which usually affect both eyes, may include:
A stinging, burning or scratchy sensation in your eyes Stringy mucus in or around your eyes Sensitivity to light Eye redness A sensation of having something in your eyes Difficulty wearing contact lenses Difficulty with nighttime driving Watery eyes, which is the body’s response to the irritation of dry eyes Blurred vision or eye fatigue
Source: Mayo Clinic
The Market for Dry Eye Disease
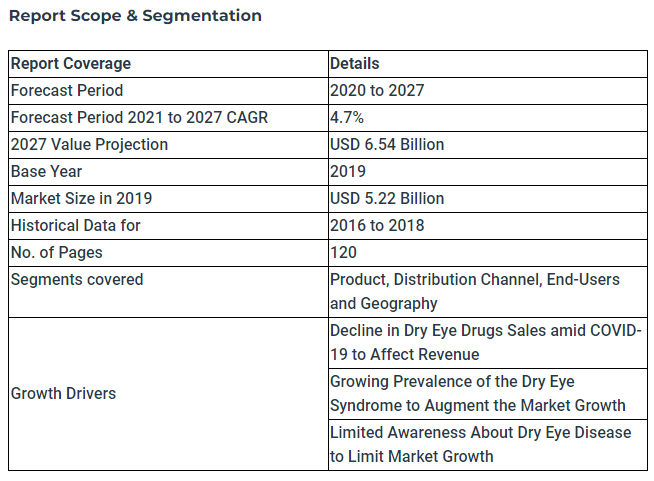
In May 2022, Fortune Business Insights recently issued a press release promoting a market research report on DED. This table from that release gives a quick snapshot of the DED market:
Fortune Business Insights
FDA Requirements for Dry Eye Disease Approval
The FDA issued draft guidance on dry eye disease in December 2020. Without getting into all the minutiae, aside from demonstrating safety, the FDA requires demonstrated efficacy in one sign and one symptom of DED over vehicle (placebo), in at least two adequate and well-controlled, multicenter independent trials.
Here is the relevant excerpt from the FDA guidance document:
• Signs of dry eye include, but are not limited to, corneal staining, conjunctival staining, decreased tear breakup time, and decreased Schirmer’s tear test score (with or without anesthesia).
• Symptoms of dry eye include, but are not limited to, blurred vision, light sensitivity, sandy or gritty feeling, ocular irritation, ocular pain or discomfort, and ocular itching. Subjects can self identify their own term for ocular discomfort, which can be used in place of any other term.
Source: Dry Eye: Developing Drugs for Treatment Guidance for Industry DRAFT GUIDANCE
One objective sign that is not specifically listed in the FDA draft document that Reproxalap has targeted is ocular redness (aka conjunctival hyperemia). This was the objective sign that was the basis of approval for EYSUVIS, a corticosteroid eye drop approved by the FDA for DED, but only short term usage.
Reproxalap Has Achieved Multiple Endpoints for an NDA and Eventual FDA Approval
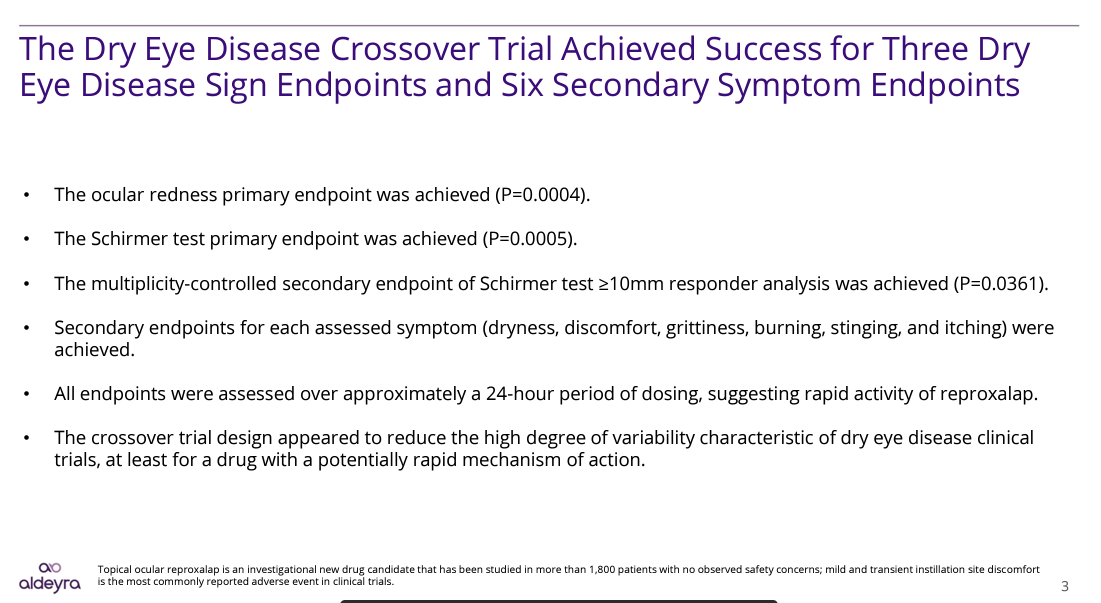
Links were provided above to some of significant data releases by Aldeyra since my initial article. However, for the sake of brevity, and to summarize the investment thesis based upon cumulative clinical data released in the last year, the focus here will be on slides presented by Aldeyra from its recent Dry Eye Disease Chamber Crossover Trial of Reproxalap presentation on July 12th. In this crossover trial, each patient acted as his/her own control, a design that eliminates patient to patient variability. After a two-week washout period, patients previously treated with Reproxalap were treated with vehicle and vice versa. I urge those interested to watch the presentation in its entirety.
The most significant takeaways from the study are presented in the slide below. Statistical significance was achieved in three DED signs and multiple DED symptoms as specified in the FDA draft guidance discussed earlier:
Aldeyra Therapeutics, Inc.
Recall that two well designed studies are required to demonstrate efficacy in each sign or symptom endpoint. As indicated in the slide below, Aldeyra now believes it is in the position to submit an NDA to the FDA requesting a label claiming efficacy in 3 signs and one symptom. The company has scheduled a pre-NDA meeting with the FDA for the current quarter ending 9/30/22. No other FDA approved DED drug has obtained a label with more than one objective sign.
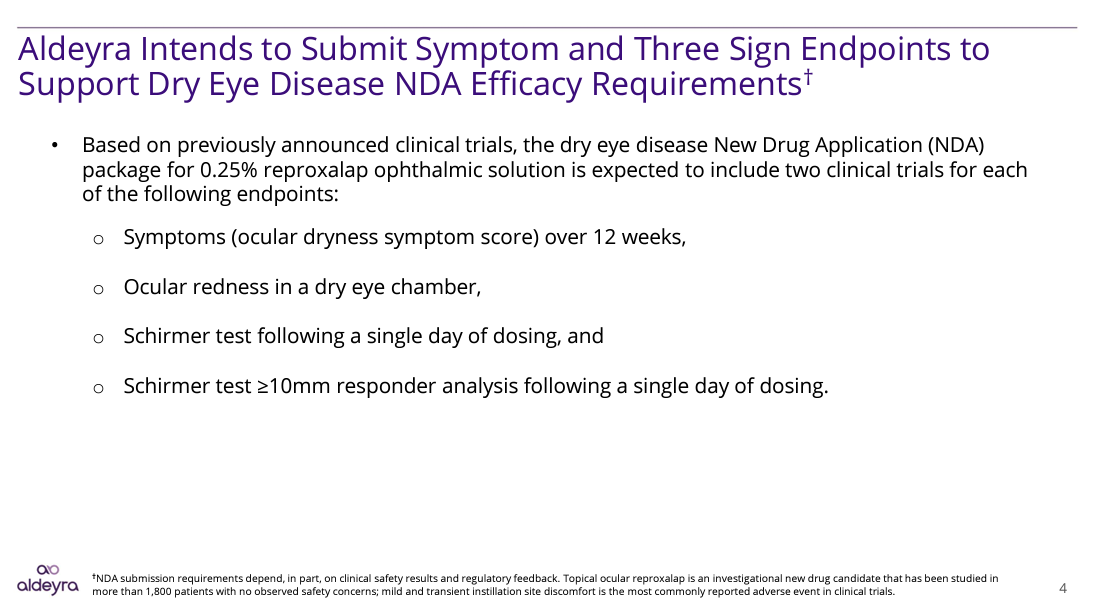
Aldeyra Therapeutics, Inc.
The sign referred to as ocular redness is self-explanatory. The two additional signs relate to the Schirmer Test, a paper wick that measures tear production.
The Big Reproxalap Advantage over the Competition – Immediate Relief to DED Patients
The market for dry eye disease includes both over the counter eye drops and prescription medications. Patients are likely to move toward prescription based medications after failing to get relief with OTC eye drops. The two leading brand name eye drop prescriptions approved for long term use are Restasis and Xiidra. In 2019, Novartis (NVS) acquired Xiidra from Takeda Pharmaceutical (TAK) for $3.4 billion at closing in a deal worth up to $5.3 Billion. In February 2022 The FDA approved the first generic version of Restasis. A nasal spray based DED drug called Tyrvaya was also recently approved by the FDA in October 2021.
Aside from being the only approved DED drop likely to achieve multiple signs on its label, in my view, the more significant advantage is that Reproxalap works rapidly. This immediate response is the reason Aldeyra was able to run a crossover trial and also represents a significant marketing advantage. Common sense dictates that, with all other considerations being equal, any rational ophthalmologist treating a patient with dry eye discomfort would naturally be inclined prescribe a drop that provides the patient with both immediate and long term relief.
In its crossover trial presentation, Aldeyra shared the following compelling studies that indicate why Reproxalap should be in a strong position to gain market share against the top players in the long term usage DED market due its rapid action advantage:
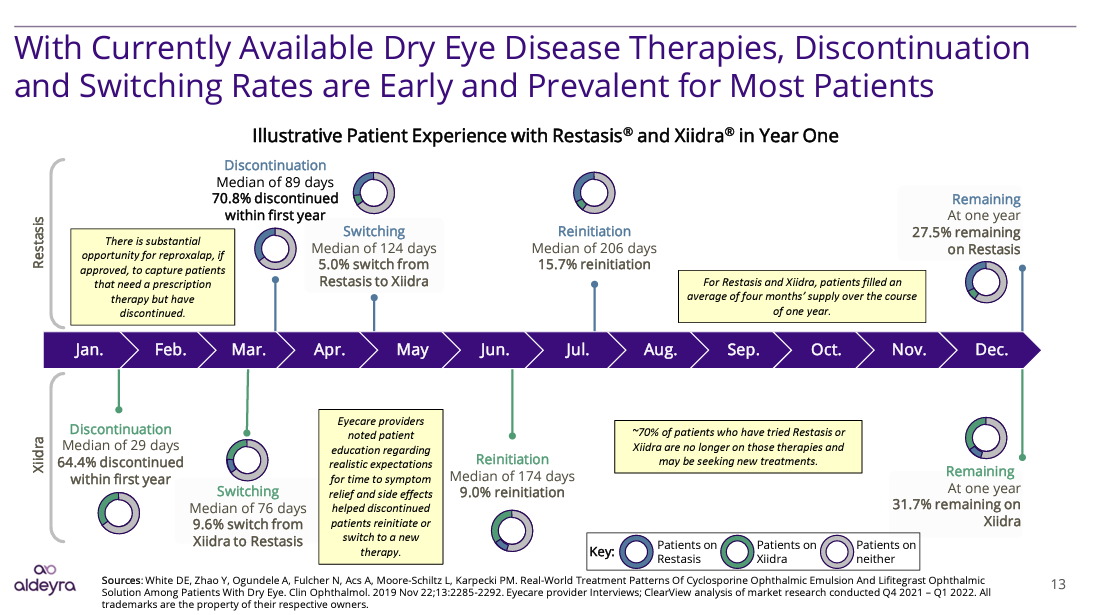
Aldeyra Therapeutics, Inc.
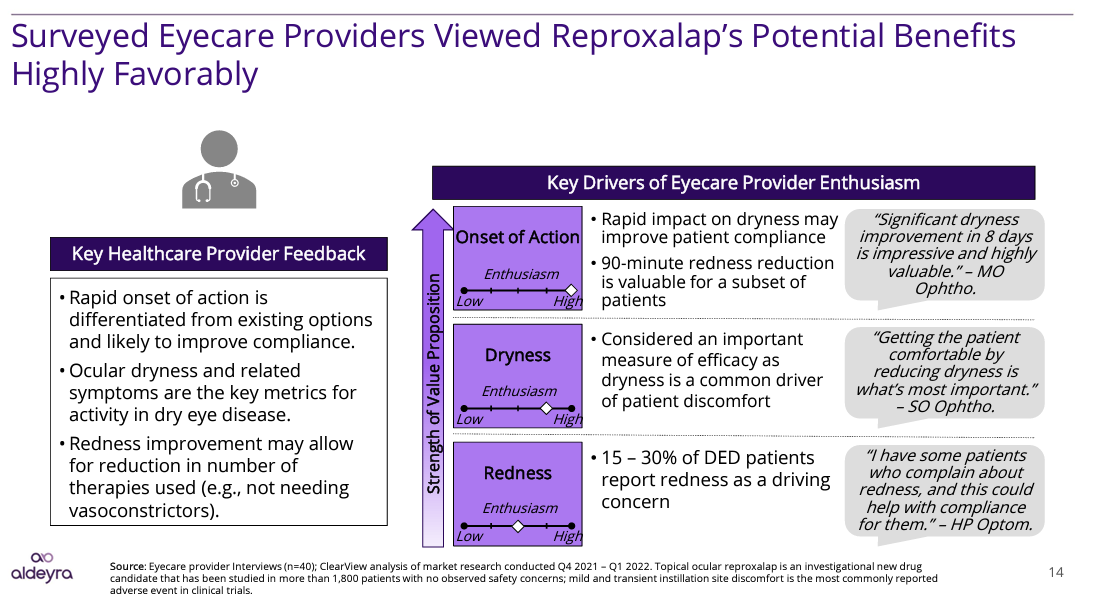
Aldeyra Therapeutics, Inc.
Current Valuation and Cash Position of Aldeyra
Like most small cap biotechs, the past year has not been kind to Aldeyra shares. At a recent price of $5.75, company has a market cap of about only $336,000,000 after peaking at $15.95 in April 2021. Unlike many small biotechs the company has a solid balance sheet as it has raised capital at opportune times. As of March 31, 2022 Aldeyra had about $217,000,000 in cash, cash equivalents and marketable securities. The company has $15,586,000 in long term debt due in October 2023 and total liabilities of $32,004,000. Cash used in operating activities, which included running multiple clinical trials, totaled $12,873,000 in the most recent quarter. The following excerpt from the company’s March 31, 2022 10-Q discloses its current cash runway…
Based on its current operating plan, the Company believes that its cash and cash equivalents, as of March 31, 2022, will be sufficient to fund currently projected operating expenses through the end of 2023, including potential new drug application (NDA) submissions; initial commercialization of reproxalap, if approved; and continued early and late-stage development of our product candidates in ocular and systemic immune-mediated diseases.
Source: sec.gov
ALDX Insider Buying
My last article on Aldeyra pointed out the insider purchases by Perceptive Advisors LLC, a 10% plus holder required to report transactions. Most of their purchases are still underwater. Recently, following the spectacular crossover results, they have been adding to their significant position:
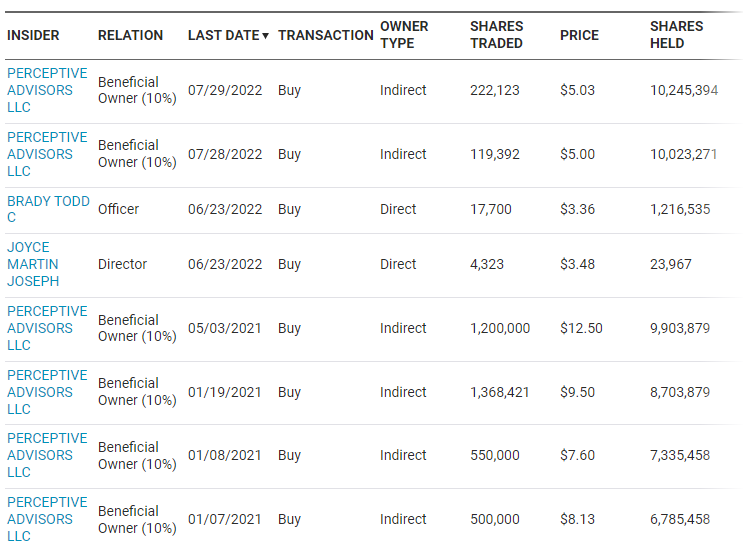
NASDAQ
Risks
The primary risk here is obvious. Despite all the positive clinical data, it is always possible that the FDA will find something it doesn’t like and could fail to accept the NDA, fail to approve Reproxalap, or delay the approval process. Aldeyra has a strong balance sheet but bringing a drug to commercialization is a complicated and expensive endeavor and any delays would be costly.
Conclusion
Aldeyra is a small company with only about 14 employees on the payroll as it outsources much of its research and development work. Although there are never any guarantees, it appears to be close to GO TIME for the FDA approval of Reproxalap for DED. However, I doubt that Aldeyra will attempt to commercialize Reproxalap on its own. A partnership or outright sale of Reproxalap seems likely. I pointed out a comparable purchase of XIIDRA by Novartis (NVS) for up to $5,000,000,000 and how Allergan recently lost exclusivity for Restasis to a newly approved generic. Allergan, now a division of AbbVie (ABBV), seems like a logical, deep pocketed partner or suitor for Reproxalap.
Aldeyra seems to be in a very enviable position and a monetization event that could include both DED and allergic conjunctivitis seems possible in the near term. In my opinion, Aldeyra shares are grossly undervalued.


Be the first to comment