Editor’s note: Seeking Alpha is proud to welcome Richard McGrail as a new contributor. It’s easy to become a Seeking Alpha contributor and earn money for your best investment ideas. Active contributors also get free access to SA Premium. Click here to find out more »

Godji10
Investment Thesis
While Novavax’s stock (NASDAQ:NVAX) has had a rough past few months, its apparent undervaluation today makes it a compelling investment. While Novavax’s currently authorized vaccine, Nuvaxovid, may not become the best-selling COVID vaccine over its mRNA competitors, its current valuation means that even if an effective small niche could be carved, NVAX stock could see significant upside. Additionally, while talked about relativity little, its flu vaccine candidate, NanoFlu, provides important potential revenue diversification and a second leg for the company to stand on.
It is no secret that the mRNA vaccines currently approved to prevent COVID-19 have a significant reactogenicity profile causing typical vaccine-related side effects like fever and fatigue. While the side effects are almost always “mild,” they can still have significant occupational and lifestyle impacts for a day or two after vaccination. For example, a study examining healthcare workers’ absence after an mRNA vaccination found that 7.4% of second dose vaccinations resulted in a short-term disability claim with higher rates in younger cohorts. Obviously, a vaccine that has lower rates of side effects would be preferable from a personal preference and economic standpoint. Novavax’s nanoparticle-based vaccine has a more favorable reactogenicity profile. Compared to Pfizer (NYSE:PFE)/ BioNTech’s (NASDAQ:BNTX) offerings, Novavax’s vaccine showed a 2.5x lower rate of fever and a lower rate of moderate-severe fatigue in its clinical trial.
Looking forward, it is becoming increasingly likely that annual boosting will be recommended as new COVID variants emerge, particularly for vulnerable populations. Because Novavax’s vaccine has similar efficacy to the currently approved mRNA options, it is my belief that people will place more emphasis on potential post-vaccine systemic side effects that could result in fever, fatigue, and potentially a missed day of work. According to Johns Hopkins, mild side effects are one of the major drivers behind vaccine hesitancy and are certainly important to a vast majority of the population who will need booster shots. Therefore, it would not be surprising at all to see a small subset of booster candidates opting for Novavax’s Nuvaxovid over its mRNA competitors. As is explored in the valuation section, even a small single-digit of market penetration in an overall bearishly-estimated declining COVID vaccine market could result in significant upside.
While not yet approved, NanoFlu could soon provide a more diverse revenue stream for Novavax outside of its currently only authorized product, Nuvaxovid. Targeting seasonal influenza, NanoFlu is extremely likely to be approved as the company is already in talks with the FDA for an accelerated approval and has received FDA fast-track designation in older adults. As of now, the seasonal flu vaccine market has been dominated by incumbent pharmaceutical giants like Sanofi (NASDAQ:SNY), GSK (NYSE:GSK), and CSL (OTCPK:CSLLY). Novavax’s NanoFlu seeks to disrupt this space with its superior nanoparticle technology that bypasses shortfalls related to egg adaptation and antigenic drift. In a phase 3 study, NanoFlu outperformed Sanofi’s FluZone Quadrivalent on measures of immunogenicity with similar safety and tolerability profiles. While seasonal influenza typically causes mild disease in most, certain populations, like seniors and the immunocompromised, are at much more risk. Therefore, NanoFlu’s superior immunogenicity could result in high penetration rates in these high-risk groups where efficacy is of paramount concern.
While this thesis seems quite straightforward, in the following valuation section, it becomes clear that NanoFlu’s potential is being missed by the market. A quick look through several sell-side research publications shows little to no mention of a stand-alone NanoFlu vaccine, instead focusing on Novavax’s COVID vaccine and a possible combination vaccine. This is despite talks with the FDA to get NanoFlu an accelerated approval and its previously discussed stellar clinical trial results. It is my opinion that the market’s focus on the coronavirus has inhibited its ability to see the upside potential with Novavax’s NanoFlu.
While Nuvaxovid and NanoFlu are the company’s two near-term vaccines that have demonstrated safety and efficacy and are already authorized or nearing accelerated approval, Novavax still has other potential vaccines (e.g Malaria and Ebola) under earlier stages of development that could prove commercially viable in the long term. While it is difficult to predict the outcomes of the respective vaccines later stage clinical trials this early, the company’s valuation, as I discuss later, still remains attractive, assuming just a “niche” Nuvaxovid and NanoFlu base case.
A Note on NVAX’s Recent Earnings and Guidance
Earlier this month, Novavax significantly cut its revenue guidance to between $2 billion and $2.3 billion, citing lower-than-anticipated COVID vaccine sales which caused the stock to drop 30%. While the market responded to this news with surprise, this actually supports the niche argument that is made within this investment thesis. While it certainly was too bullish to assume that Novavax would cannibalize significant market share from its incumbent competitors, a more conservative approach of acquiring single digits of market share with both its COVID and Flu offerings is much more realistic. As is seen below in the valuation section, an estimate even more conservative than management’s projections could still result in significant upside.
Valuation
The table below represents a conservative estimate for a successful niche vaccine deployment in both Nuvaxovid and NanoFlu products. This conservative estimate is intentional because it shows that even if Novavax does not achieve management or analysts’ expectations, there is still significant value to be had (current analyst consensus is >$2 billion in 2026 revenue while management expects a 20% Nuvaxovid market share). The projections below imply an 8% Nuvaxovid market penetration and a total revenue of <$1.5 billion.
On the NanoFlu front, its penetration rates are roughly based upon cannibalizing a substantial portion of Sanofi’s FluZone High-Dose Quadrivalent market share (Sanofi’s offering for high-risk populations). Additionally, the following estimates also take into account the market’s fear of declining annual COVID vaccination numbers. Despite the bearishly estimated overall COVID vaccine market and the intentionally conservative Nuvaxovid market share projections (due to relative uncertainty), Novavax’s stock still seems undervalued.
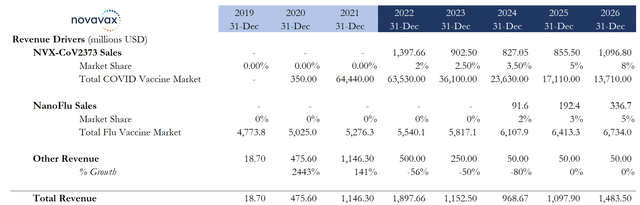
Statista, company data, my estimates
While I typically utilize discounted cash flow models to value prospective investments, a more creative valuation technique is required for Novavax. This is because a proper DCF requires WACC = ROIC in the terminal year. Due to the early-stage nature of Novavax, I do not foresee WACC closely approaching ROIC anytime soon, so detailed projections would need to be made 10+ years into the future. Projections this far into the future must assume far-off clinical trial results, among other things, and are not terribly valuable; therefore, I am valuing Novavax here based on a forward revenue multiple compared to a similar comps group. I decided on the year 2026 to use for the multiple because annual COVID vaccination numbers will likely begin to stabilize by then and NanoFlu would have had at least one or two flu seasons under its belt to become a more established player.
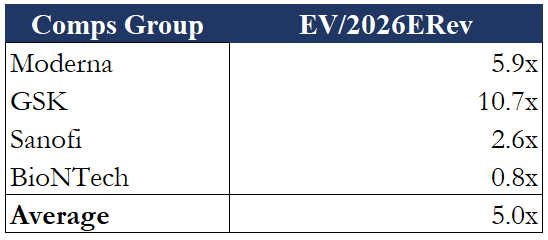
Company Filings
Assuming $1.484 billion revenue in 2026, this implies that NVAX trades at an EV/2026ERev multiple of 1.55x while a comps group of Moderna (NASDAQ:MRNA), Sanofi, GSK, and BioNTech trades at an average of 5.0x (sell-side estimates). Applying this valuation and a somewhat conservative case to Novavax would imply huge amounts of upside. Some will be quick to point out, however, that all the other companies in this comps group have other promising non-infectious disease therapies in the pipeline that demand a higher valuation; therefore, they would argue that 5.0x might be difficult for NVAX to reach. In my opinion, however, this somewhat bullish multiple is balanced out by the conservative nature of Novavax’s revenue estimates made earlier. Furthermore, while NVAX may not be targeting cancer or other diseases like its competitors, the company still has value in its pipeline with the previously mentioned vaccine candidates (e.g., Malaria and Ebola) and a proven vaccine development platform that should also demand a higher valuation.
If NVAX were to realize its comps group average EV/2026ERev multiple of 5.0x in the future, its share price would likely return to its previous triple-digit levels.
Catalysts/Risks
While some may view the Biden administration’s refusal to purchase further COVID vaccines as a risk, in reality, it could play to Novavax’s advantage and act as a long-term catalyst. This is because Novavax’s success in the COVID vaccine space requires them to make an appeal to a small subset of the population, as discussed in the first part of the investment thesis. Rather than needing to assert their value proposition to the government, healthcare providers, and potential recipients, Novavax can just focus on the latter two. This could potentially allow them to carve out their niche more effectively.
Additionally, full approval of Nuvaxovid down the road could increase adoption rates as people begin to see Novavax’s offering as equal, or possibly even better, to the currently approved mRNA options. As discussed more in-depth in the investment thesis, the superior reactogenicity profile of the Novavax vaccine combined with a full FDA approval could dramatically increase Nuvaxovid sales. Assuming a similar timeline to the previously approved vaccines, an approval of Nuvaxovid can be expected sometime around Q2 2023, plenty of time before the next annual booster campaign would begin.
Conclusion
While many see Novavax as being unable to effectively compete with the already established players in the COVID and flu vaccine space, the recent decline in Novavax’s stock price has finally made its valuation attractive for this “niche” penetration case discussed within the investment thesis. While risks still remain regarding Novavax’s concentration in just two products and the public’s perception of Nuvaxovid, the company’s valuation compared to its established competitors makes it an extremely compelling investment, and a return to its previous triple-digit levels is certainly possible.


Be the first to comment