juststock
Thesis
BioVie (NASDAQ:BIVI) has gained considerable attention since the favorable preliminary data relating to NE3107’s treatment for Alzheimer’s disease in Phase 2 clinical trials. The multiple preclinical and clinical trials, including recent clinical data, set optimistic expectations for NE3107’s future clinical trial results and its eventual approval for the common neurodegenerative diseases Alzheimer’s and Parkinson’s. The NE3107 clinical candidate is further supported by BIV201, which remains highly de-risked, given the drug’s active ingredient has been approved in European countries and, more recently, in the U.S. BIV201’s administration mechanism, which is continuous infusion, has been studied by global investigators and generally found to have a favorable profile. Both candidates are being developed on a solid foundation, with the company estimating U.S. peak sales potential for NE3107 at $8.5 billion and BIV201 at $450 million.
6 months share price (ycharts)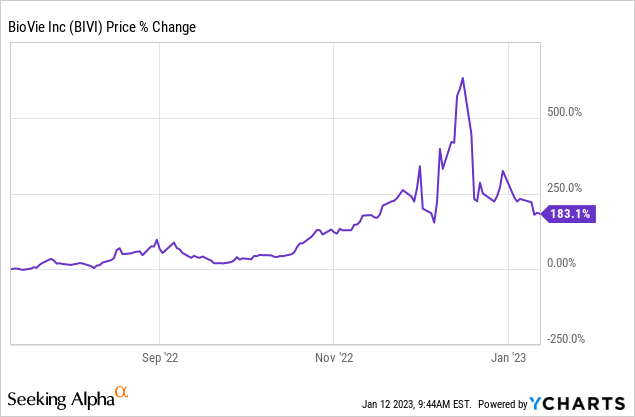
Company Overview
BioVie Inc. is a clinical-stage biotechnology company concentrating its efforts on developing novel therapies for multiple neurodegenerative disorders and refractory ascites. The company’s lead therapeutic candidate, NE3107, is currently being evaluated in a Phase 3 clinical trial for Alzheimer’s disease (AD) and in a Phase 2 trial for Parkinson’s disease (PD). The novel small molecule was part of BioVie’s acquisition of NeurMedix, Inc.’s biopharmaceutical assets in a stock/cash deal that concluded in June 2021. Furthermore, the company’s follow-on pipeline candidate, BIV201, holds orphan drug status and could treat ascites and other complications arising from liver cirrhosis. The drug candidate is now being examined in the Phase 2b clinical trial for refractory ascites.
Company presentation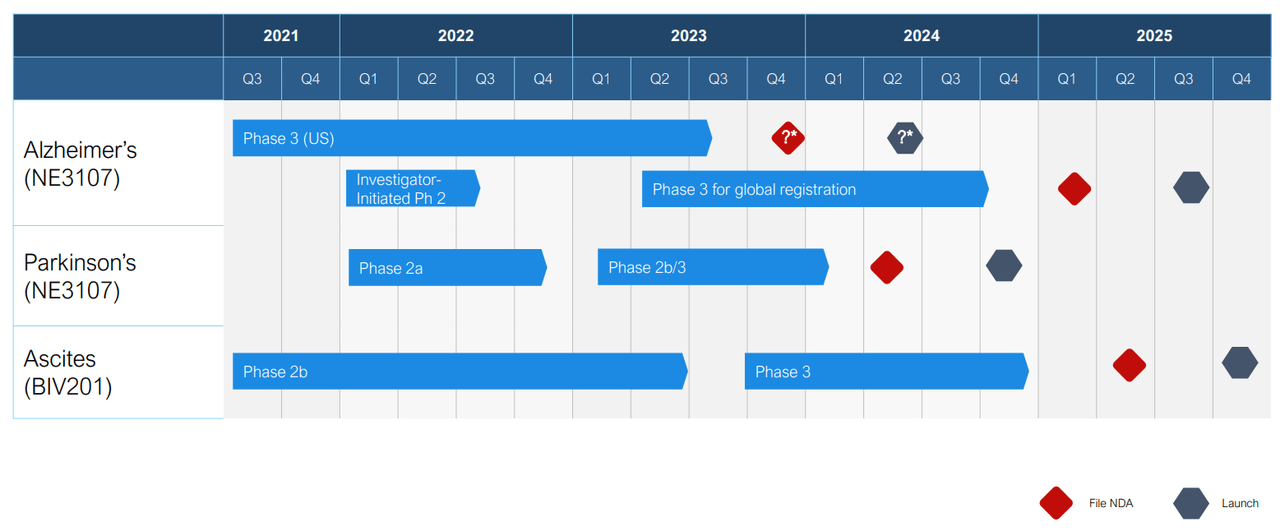
Exhibit 1: Anticipated Timeline and Major Catalysts.
An Introduction to Neurodegenerative Disorder
Neurodegenerative diseases (NDDs) are generally characterized by the gradual deterioration and death of neurons, the building blocks of the nervous system. Neuronal cell degradation and death are often followed by impairment of sensory, motor, and cognitive processes. Neurodegenerative disorders are highly complex and can have different pathogenesis pathways or shared mechanisms of neuro-degeneration. NDDs are distinguished as Alzheimer’s disease, amyotrophic lateral sclerosis, Huntington’s disease, Parkinson’s disease, etc., depending on the part of the central nervous system (CNS) tissue affected that leads to worsening of neurological symptoms.
Common Mechanism of Nero degeneration (chart- Molecular Neurodegeneration)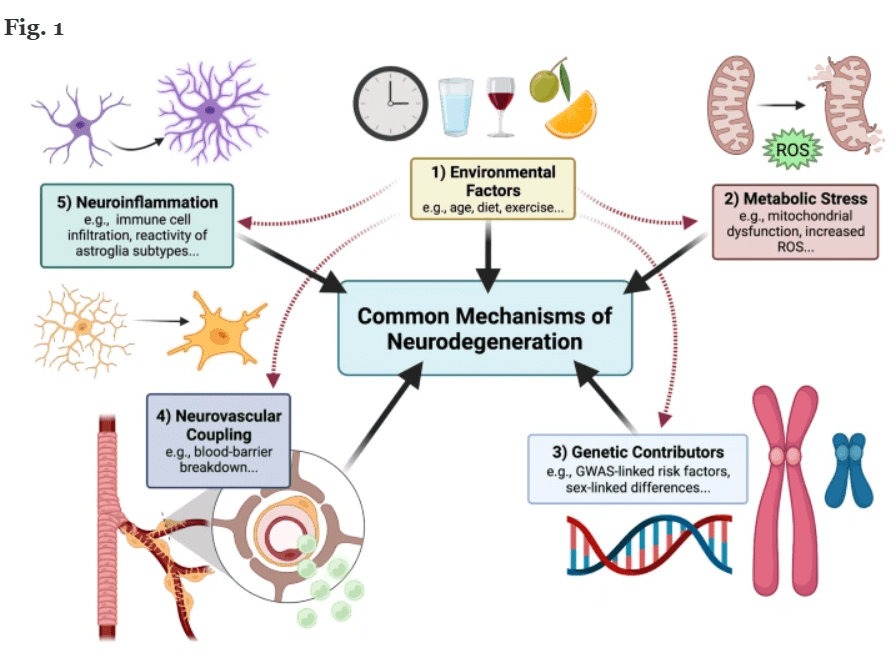
Exhibit 2: Common Mechanism of Neuro-degeneration.
Understanding the pathways and mechanism to neuro-degeneration while identifying similarities across different NDDs holds the key to developing a pan-neurodegenerative therapeutic approach.
Alzheimer’s disease (AD) is one of the most common neurodegenerative disorders, with a global incidence of more than 50 million people that is expected to more than double by 2050. Alzheimer’s is a chronic, debilitating disease with catastrophic effects on cognitive processes, typically leading to dementia. The disease has majorly been characterized by the accumulation/aggregation of amyloid-β plaques and intracellular neurofibrillary tangles (NFT) of tau across the affected area of the brain with a massive loss of neurons and shrinkage of the hippocampus.
Comprehensive Review on Alzheimer’s Disease: Causes and Treatment (Molecules, 2020)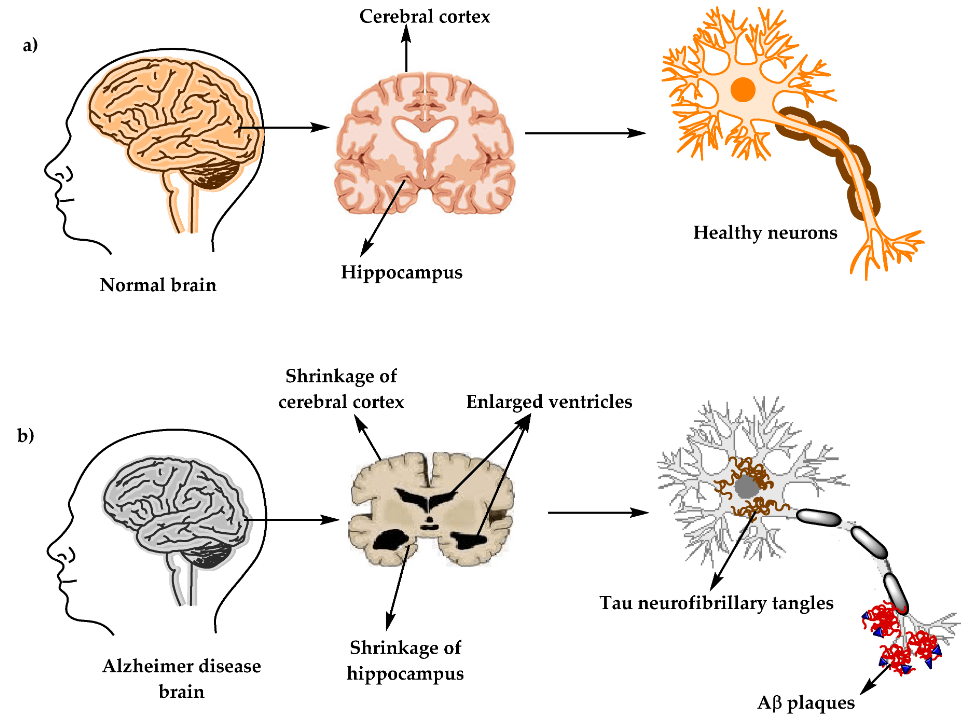
Exhibit 3: Comparison of the Physiological Structure of a Healthy Brain and Alzheimer’s Disease Brain.
The most widely accepted hypotheses of pathogenic mechanisms in Alzheimer’s disease remain the amyloid cascade and tau hypotheses. Even though both hypotheses have been widely accepted as the primary hallmarks of AD, limited success has been achieved in developing drugs based on them and their ability to explain the neuro-degeneration in AD. Given the complex and elusive nature of the disease, drugs approved until now (rivastigmine, galantamine, donepezil, and memantine) are symptomatic treatment approaches and do not prevent disease progression, although they have been successful in demonstrating modest improvement in cognition and function. The FDA’s approval of aducanumab, the first new Alzheimer’s drug in more than 18 years, had attracted some skepticism from the scientific community regarding its efficacy in treating AD. Biogen’s aducanumab, marketed under the brand name ADUHELM®, attempts to reduce the aggregation of amyloid-β plaques to try to treat neuro-degeneration. The 2021 approval of ADUHELM® is followed by the FDA’s recent accelerated approval of Biogen’s other anti-amyloid mAb, lecanemab. Both drugs are based on the amyloid cascade hypothesis.
It is becoming clearer that the neurodegenerative mechanism of AD can’t just be explained by the two most widely accepted abnormal factors, tau and Aβ. Given the hypothesized multifactorial nature of the disease, increasing research is also being focused on other possible mechanisms and influences, including inflammation, insulin resistance, vascular effects, oxidative stress, etc.
Alzheimer’s Disease Pathway (company presentation)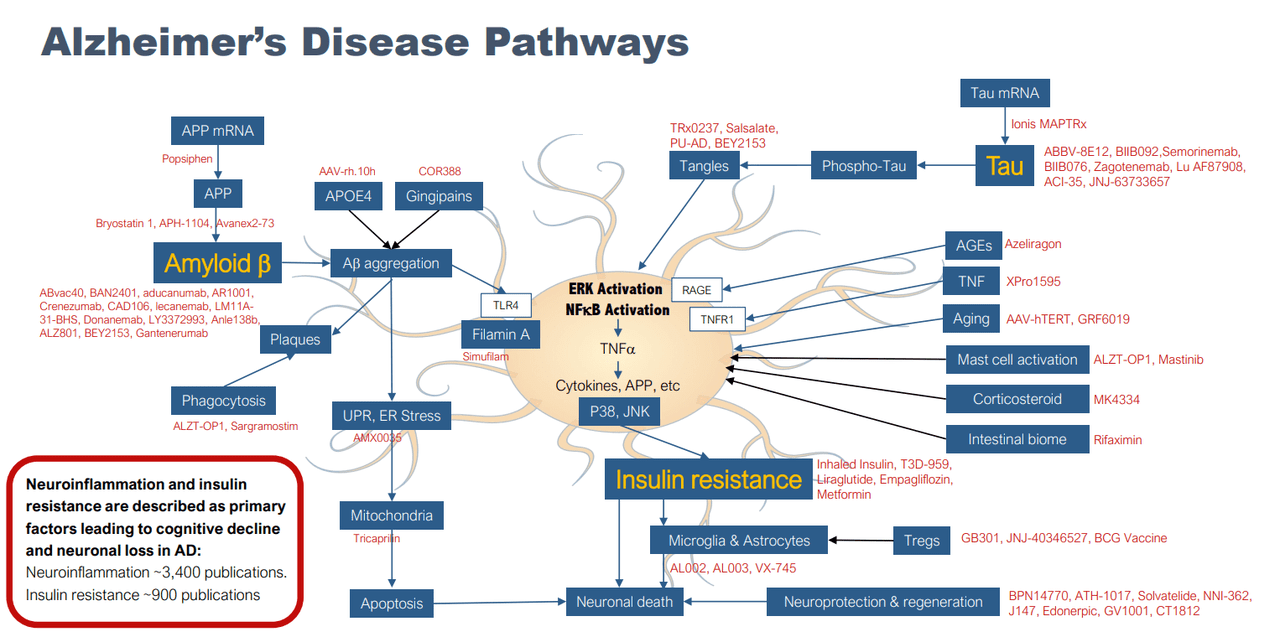
Exhibit 4: Alzheimer’s Disease Pathway.
Parkinson’s disease (PD) is another common neurodegenerative disorder characterized by the gradual degeneration of dopaminergic neurons, typically leading to motor control abnormalities. Similar to Alzheimer’s, PD is also considered to be a multifactorial disease with a pathogenesis influenced by various factors, including genetic and environmental. In addition to the loss of dopaminergic neurons, the misfolding and aggregation of alpha-synuclein, a key neuronal protein, is considered a key molecular hallmark for the pathogenesis of PD. Treatment strategies focusing on the inhibition of alpha-synuclein aggregation, alpha-synuclein vaccines, and LRRK2 strategies (The LRRK gene has been found to regulate inflammatory responses and the transmission of alpha-synuclein.) are undergoing extensive research to potentially change the course of the disease, but the research has had limited success so far. Other important pathogenic mechanisms that can be explored and leveraged to develop treatments include mitochondrial dysfunction, neuro-inflammation, a dysfunctional protein clearance system, etc.
Traditional therapeutic strategies have long focused on hallmark pathogenic pathways but have had limited success in modifying the disease course. Both major neurodegenerative diseases, Alzheimer’s and Parkinson’s, have different pathogenic pathways, but neuroinflammation and insulin resistance, to an extent, have been identified as playing common and key roles in the pathogenesis of both.
In the event of brain tissue damage or infection, microglial (immune cells in the brain) activation releases inflammatory cytokines and communicates with other immune cells in the brain, including astrocytes, dendritic cells, neutrophils, and monocytes. Both microglia and astrocytes are part of the brain’s innate immune system, which promotes the release of both pro-inflammatory (IL-1β, TNF-α, IL-6) and anti-inflammatory (IL-4, IL-10) cytokines that not only lead to tissue repair but also play a central role in neuronal damage. In neurodegenerative diseases like AD and PD, over-activation of microglia often leads to increased creation and release of pro-inflammatory cytokines, resulting in neuronal damage. Studies have also found that microglial activation plays a key role in the aggregation and presence of misfolded proteins in the brain and further cascading effects. Recent studies have shifted much of the focus toward chronic neuro-inflammation as a key pathogenic pathway in treating multiple neurodegenerative diseases. Much research is now concluding that neuro-inflammation precedes the presence of misfolded proteins, and eliminating these proteins would not necessarily change the course of neurodegenerative disease. Additionally, in the case of AD, Aβ plaques were also discovered in healthy brains showing no symptoms of AD, which raises questions about their causative ability.
NE3107: Treating Multiple Neurological Conditions
NE3107 is BioVie’s lead candidate currently being evaluated in a Phase 3 clinical trial for mild-to-moderate Alzheimer’s disease. NE3107 is an oral, small-molecule agent with the ability to cross the blood-brain barrier and potentially inhibit multiple inflammatory mediators. The drug has been a part of multiple preclinical and clinical trials and has successfully exhibited the ability to inhibit inflammatory cascades and insulin resistance without any indication of an impaired immune response.
NE3107’s Mechanism of Action (MoA)
The company’s lead drug candidate utilizes dual pathogenic pathways — neuro-inflammation and insulin resistance — to target both Alzheimer’s and Parkinson’s. NE3107 inhibits the ERK cell signaling pathway and transcriptional factor activation of nuclear factor kappa B (NF-κB) as a mechanism for reducing neuro-inflammation.
The extracellular, signal-regulated kinase pathway (ERK pathway) is responsible for numerous cellular functions, including homeostatic activity and inflammation. Multiple studies have concluded that the ERK signaling pathway plays a role in the neuronal cell death that occurs in multiple neurodegenerative diseases, including AD. The nuclear factor kappa B (NF-κB) is a protein complex that plays a vital role in regulating the production of TNFα, which is considered the master regulator of inflammation and the starting point for the expressions of pro-inflammatory cytokines and chemokines responsible for neurodegeneration. TNF-α is believed to be the master inflammatory biomarker playing a major role in chronic regulation in the progression of AD. A research study analyzing the electronic records of 56 million patients found a high correlation in treatment with tumor necrosis factor (TNF) blocking agents and a lower risk of AD in patients with arthritis and psoriasis, indicating the role of TNF-α in regulating neuroinflammation. The company’s approach stands on decades of scientific research and is akin to being an orally available, safe anti-TNF-α therapy that can potentially slow down cognitive decline and progression of neurodegenerative disorders.
Both NF-κB and ERK pathways are important regulatory mechanisms of inflammatory cascades in neurodegenerative disease. NE3107 modulates the ERK pathway and its activation of NF-κB transcription factors, reducing the creation and release of TNF-α, which is responsible for the cell-signaling events that lead to apoptosis or necrosis. This mechanism accounts for the non-immunosuppressive nature of NE3107, unlike other TNF-α inhibitors and unlike ERK blockers.
NE3107 (Reading, C.L. et al. 2021)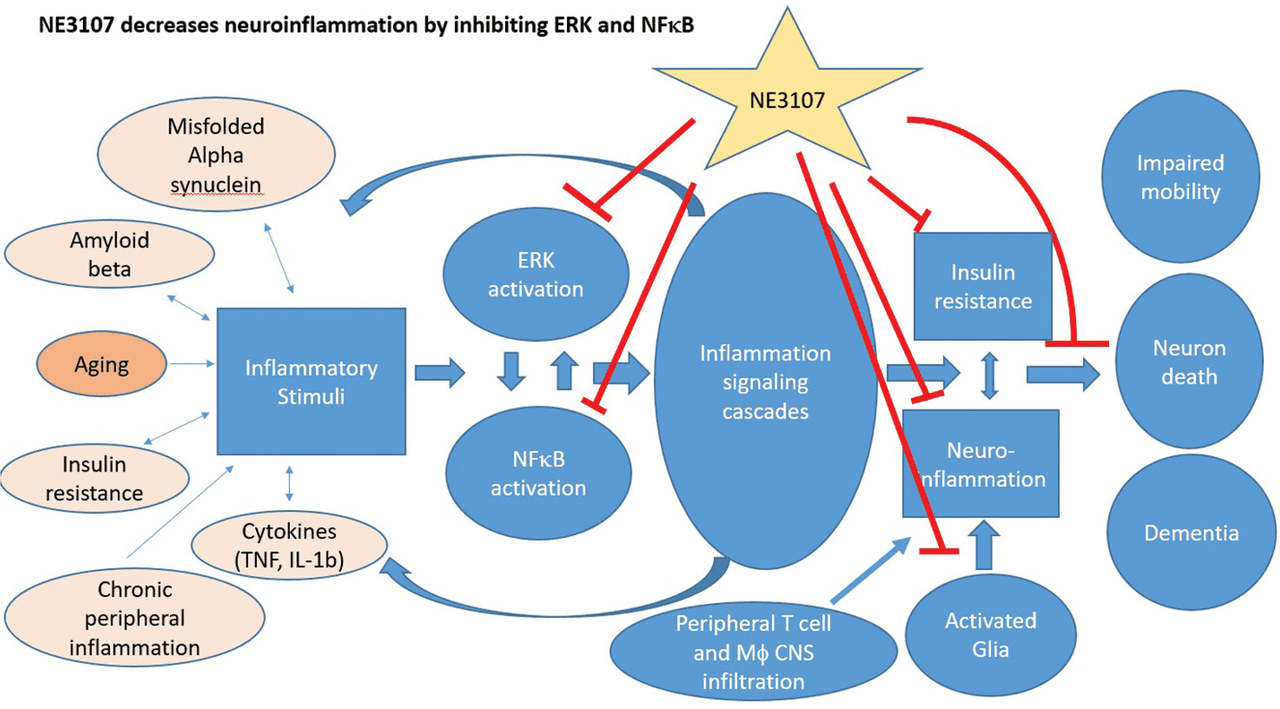
Exhibit 5: NE3107 MoA.
Insulin resistance is another crucial pathogenic pathway in AD and PD, and in combination with Inflammation, it has been found to contribute to the aggregation of misfolded proteins, tau, and amyloid plaques with deteriorating nerve stimuli. Further, type 2 diabetes and AD are highly linked, given the commonalities in their characteristics, including impaired glucose tolerance and oxidative stress. Multiple studies (Molecular Psychiatry, 2022, Expert Opin Investig Drugs, 2020, Diabetes July 2014) have shown the merits of anti-insulin-resistance therapies and improved insulin signaling as a means to restore cognitive impairment and promote neuron survival.
Discussions of Clinical and Preclinical Trial Results
NE3107 has been part of multiple clinical and preclinical studies, with 178 subjects being exposed to at least one dose of the drug or a placebo. According to the studies, there were no differences in adverse event rates between the treatment and placebo arms, and the drug was well tolerated. The majority of the reported adverse events were mild to moderate and were not related to NE3107.
NE3107 was also evaluated under multiple preclinical trials, including the rat glaucoma model, optic neuritis, and the mouse and marmoset Parkinson’s model. The data indicated improved insulin signaling and decreased postprandial blood glucose levels. NE3107 was able to regulate pro-inflammatory biomarkers, including IL-6, IL-17, IL-23, IFN-γ, and TNF-α, while restoring homeostatic functioning. The neuro-protective benefit was visible majorly in preclinical models where NE3107 was able to reduce inflammation and microglia activation. The Parkinson’s mouse and marmoset models exhibited a decrease in pro-inflammatory biomarkers such as iNOS and TNF-α by 20% and 40%, respectively. These results were further accompanied by a 17% increase in the number of dopaminergic (TH-positive) neurons.
The company recently announced the results of its exploratory biomarker and imaging trial evaluating NE3107 at a dosage of 20mg twice daily for three months in patients with mild-to-moderate (n=18 to n=5) AD. The initial results support NE3107’s targeted dual-pathogenic pathway, inflammation and insulin resistance, in treating AD. The drug appeared to be well tolerated and safe, with no drug-related adverse events.
CTAD Abstract CTAD Abstract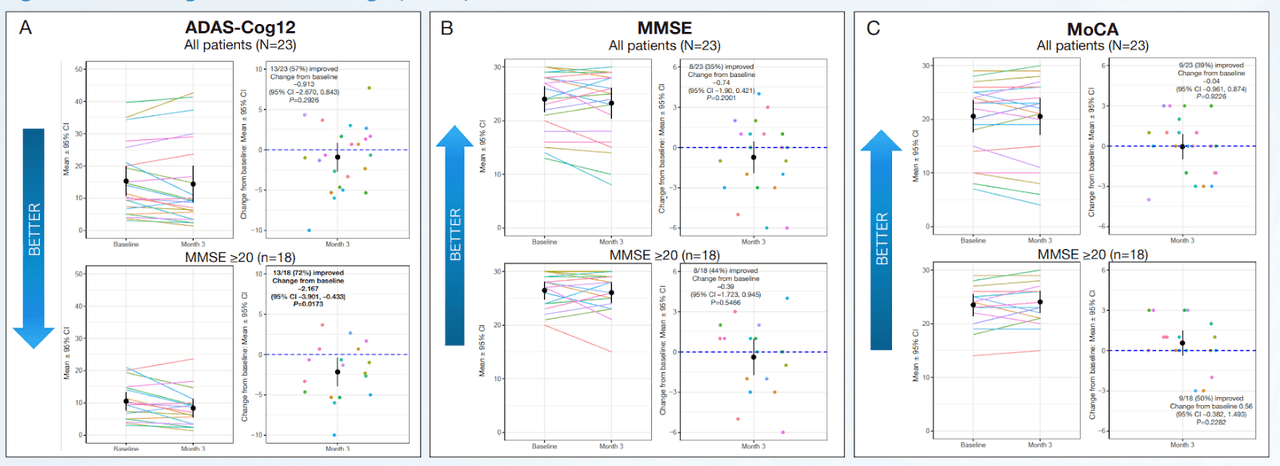
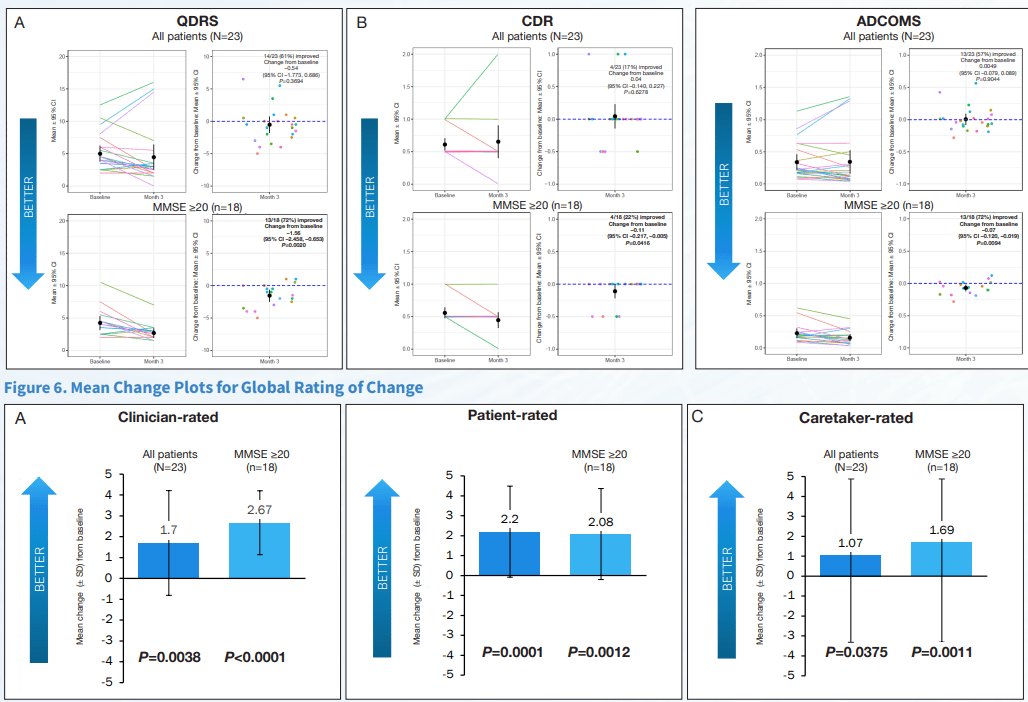
Exhibit 6:Cognition and Function
The important clinical features of AD, cognition and function were measured using the ADAS-Cog12 score and global rating of change. NE3107 exhibited improved cognition as measured by multiple cognitive assessment tools, including a 2.2-point or 21.1% improvement from the baseline on the ADAS-Cog12 scale, a 0.11-point or 19.4% improvement from the baseline on the CDR scale, and a reduction in the ADCOMs score indicating improved cognition. Similar results were observed using the GRoC scale, with the majority of patients reporting improved functioning. Furthermore, more than 60% of mild AD patients exhibited a reduction in TNF-α, indicating NE3107’s anti-inflammatory actions. NE3107 also induced a reduction in the concentration of CSF AD biomarkers p-tau and a reduction in the ratio of p-tau to Aβ42. Fifty-nine percent of the mild AD patients displayed increased levels of brain glutathione (a master antioxidant) from the baseline. The trial supported NE3107’s efficacy with a broad set of favorable outcome measures, including imaging that showed normalization of the hyperactive hippocampus. The cognition and function tests supported by the biomarker and imaging data all indicated a favorable efficacy profile for NE3107 and highlighted the neuro-inflammation pathway in AD pathogenesis. Although multiple clinical trials have exhibited NE3107’s safety and anti-inflammatory, anti-insulin-resistant properties, none had previously been focused specifically on human clinical neurodegenerative disorders. This recent Phase 2 exploratory biomarker and imaging trial in AD provides the initial confirming evidence of NE3107’s potential to improve cognition and function and exhibit neuro-protective benefits.
Inflammatory and CSF AD Biomarker Evaluation Source: CTAD Abstract CTAD Abstract CTAD Abstract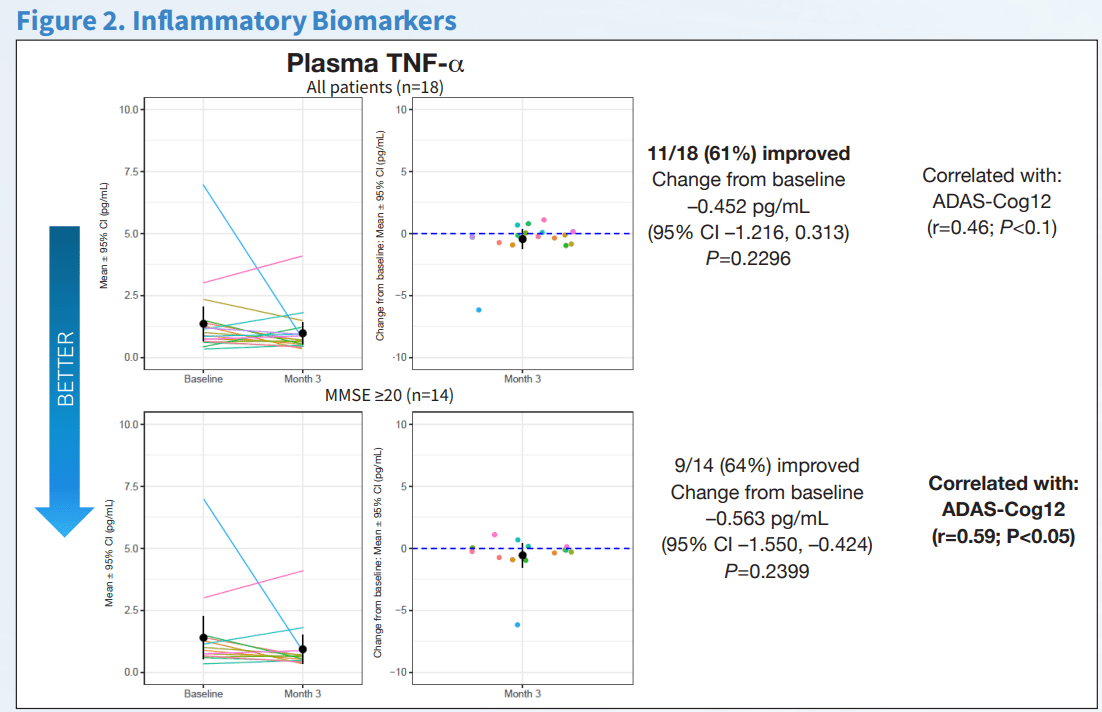
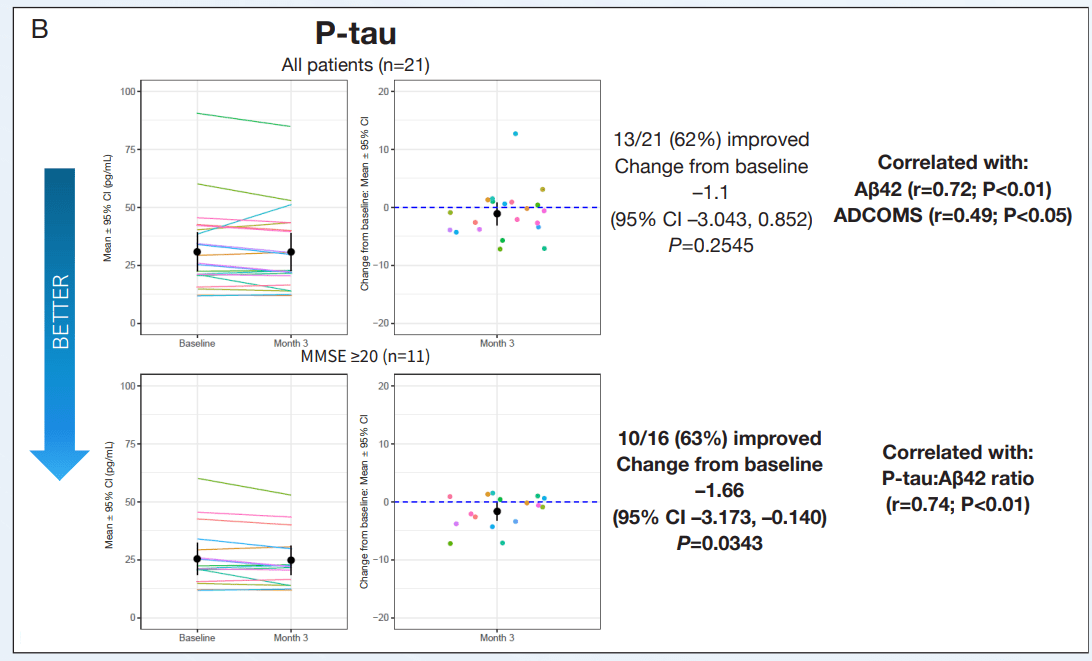
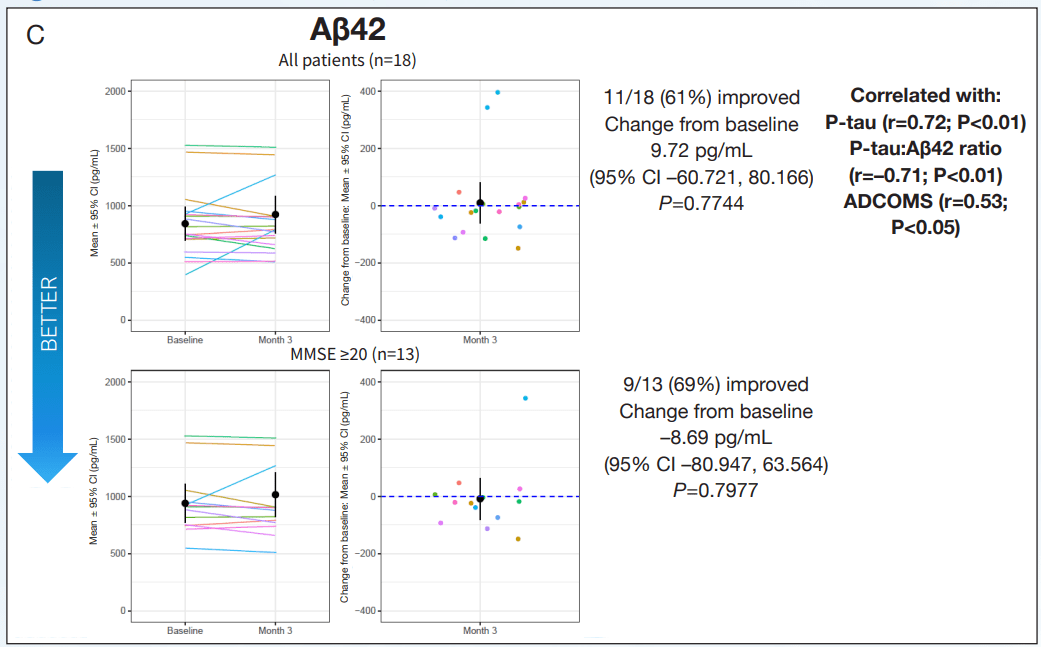
Exhibit 7: Inflammatory and CSF AD Biomarker Evaluation
BioVie is currently evaluating NE3107 in a Phase 3 clinical trial in patients with mild-to-moderate AD. The company had initially planned to enroll 316 patients. It concluded the enrollment before 50% of the enrolled patients had completed the study, enabling an optional interim review to adjust the sample size. Based on the robust top-line results in Phase 2 and the lack of adverse events, BioVie will now enroll an additional 84 patients. The company is expected to enroll up to 400 patients in total, with the top-line results expected by the end of Q3 2023.
In a recent press release, the company announced an important finding from its Phase 2 AD clinical trial. Based on blood samples taken from the patients enrolled in the trial, it was found that NE3107, in addition to improving cognition and function, could alter DNA methylation associated with epigenetic biological clocks. The data indicated a reduction of 3.3 years on the Horvath DNA-methylation SkinBlood clock. The initial findings suggest more research is warranted on NE3107’s impact on the biomarkers of aging-related disease states and its relation to the progression of AD.
Positive Results from the Phase 2 Parkinson’s Trial
NE3107 showed similar promise and improved motor control in a pilot study in patients with PD. In combination with levodopa, NE3107 showed improved motor control compared to levodopa alone, as exhibited by the Unified Parkinson Disease Rating Scale (UPDRS). The initial results demonstrated meaningful clinical benefit based on the UPDRS part 3 scale, with the superiority of the combination magnified in patients younger than 70 years old, as indicated by a more than six-point improvement on the scale. The trial indicated no adverse events and displayed superior efficacy signals of improved motor function with the NE3107-and-levodopa combination. The company will present the complete data at the AD/PD™ 2023 International Conference on Alzheimer’s and Parkinson’s Diseases, to be held March 28-April 1, 2023.
company presentation Initial Clinical Trial Results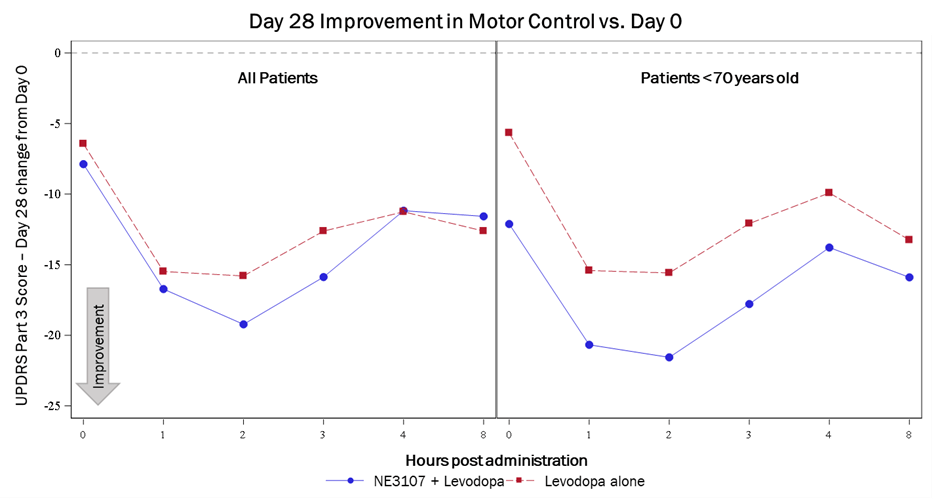
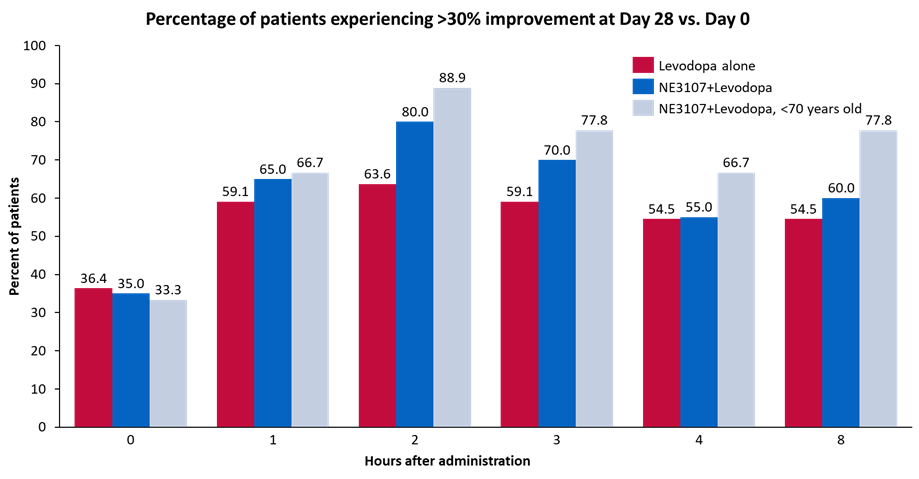
Exhibit 8: Initial Clinical Trial Results
BIV201: Potential Treatment For Refractory Ascites
BIV201 is BioVie’s novel drug candidate being developed as part of its liver cirrhosis program, with the primary indication being refractory ascites. The underlying drug became part of the company’s product pipeline through the acquisition of LAT Pharma LLC and the subsequent rights to its BIV201 therapy.
The BIV201 therapy is based on the active ingredient terlipressin, a pharmacological agent which is the analog of the hormone vasopressin and a potent vasoconstrictor. Terlipressin is approved across 40 countries for complications arising from ascites and has been recently approved by the U.S. FDA as the only terlipressin drug for the treatment of hepatorenal syndrome (HRS). BioVie was granted orphan-drug status and fast-track designation for BIV201 for ascites (primary indication) and hepatorenal syndrome.
Refractory Ascites – A Disease with Unmet Medical Needs
Refractory ascites is a complication arising from cirrhosis of the liver, leading to the accumulation of fluid in the abdominal spaces. Ascites is a serious condition indicating liver damage that causes abdominal pain, fatigue, shortness of breath, and abdominal infections, also potentially leading to kidney failure. Refractory ascites is accompanied by an adverse prognosis with a one-year survival rate of less than 50%. An estimated 20,000 patients in the U.S. are diagnosed with refractory ascites every year.
There is no FDA-approved medication for the condition. Treatment options include paracentesis (removing excess fluid by inserting a needle into the abdomen) and, in the worst-case scenarios, a transjugular intrahepatic portosystemic shunt (TIPS, or inserting a stent) and liver transplant. Another treatment, implanting a device known as the Alfapump® system, has already been approved in Europe and is currently in clinical trials for potential approval from the U.S. FDA for refractory ascites.
BIV201: Mechanism of Action
company website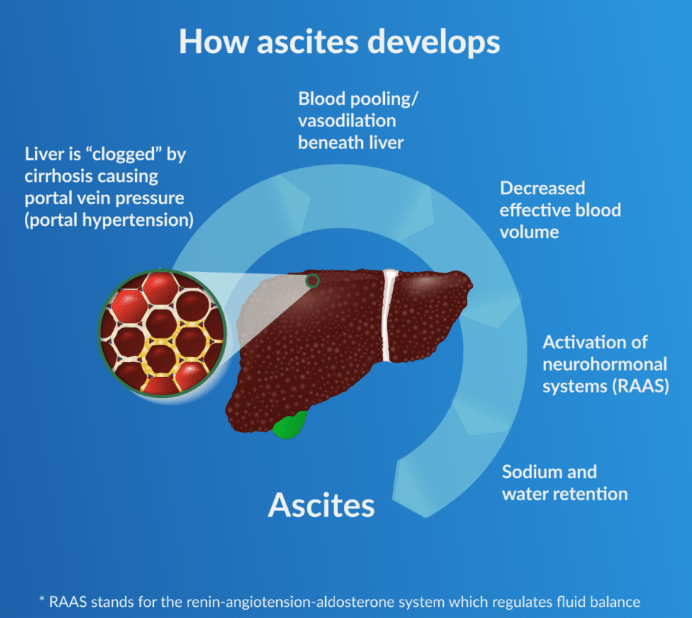
Exhibit 9: Ascites Development Pathway.
“Many experts concur that ascites development follows the sequence illustrated above. High levels of blood pressure in the vein supplying blood to the liver are observed as increased liver damage (cirrhosis) hinders blood flow through the organ. This results in vasodilation and blood pooling within the central/splanchnic area of the body and low blood volume in the arteries. The decline in effective blood volume initiates a signaling pathway referred to as “neurohormonal systems,” which then causes the kidneys to retain high levels of salt and water in order to increase the blood volume. The retention of excessive sodium and water causes ascites as the substance leaks from the liver and lymphatic system and pools in the abdominal region.” – Rephrased from BioVie’s 10K filing.
BIV201 aims to break the cycle of excessive fluid retention by alleviating portal hypertension and correcting vasodilation, eventually improving blood flow and increasing blood volume, thus reducing water and salt retention signaling to the kidneys. BioVie has modified terlipressin and invented a proprietary novel formulation, which is being administered in Phase 2b clinical trials as continuous IV infusions. The company aims to enable outpatient administration. BioVie has been granted a patent until 2036 for BIV201 (continuous-infusion terlipressin) as a monotherapy treatment for patients diagnosed with refractory ascites.
Discussion of Clinical Trial Results
The Phase 2a clinical trial included patients with refractory ascites and hepatorenal syndrome (HRS) who are using a continuous pump. The study demonstrated a decrease in ascites fluid and improved renal function in a readout that included six patients treated with a continuous infusion of terlipressin. A 46% decrease in the frequency of paracentesis treatment and a 71% to 414% increase in the number of days between paracentesis treatments were observed after treatment with a continuous infusion of terlipressin.
company presentation company presentation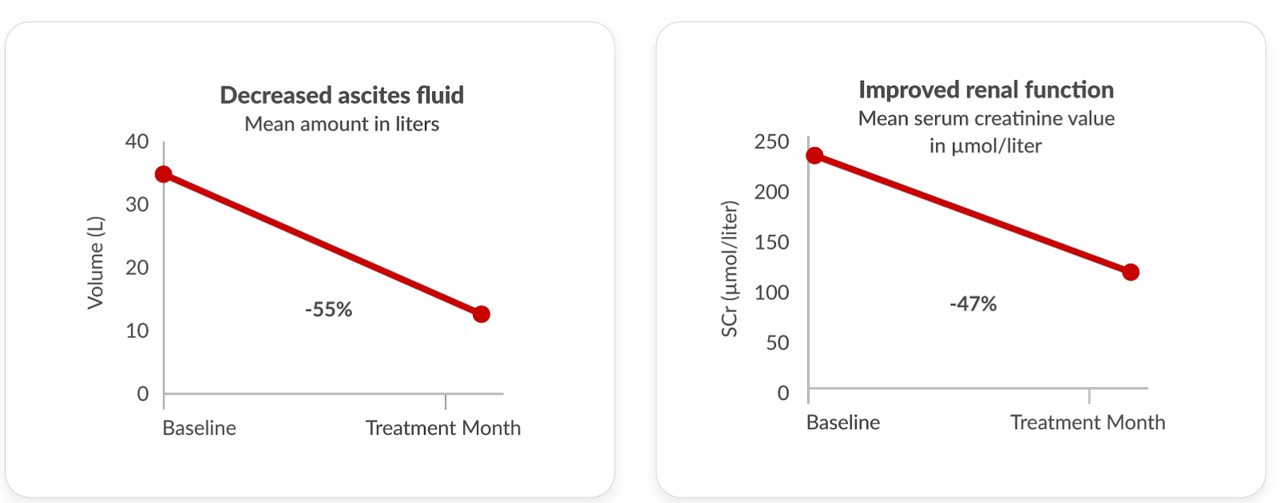
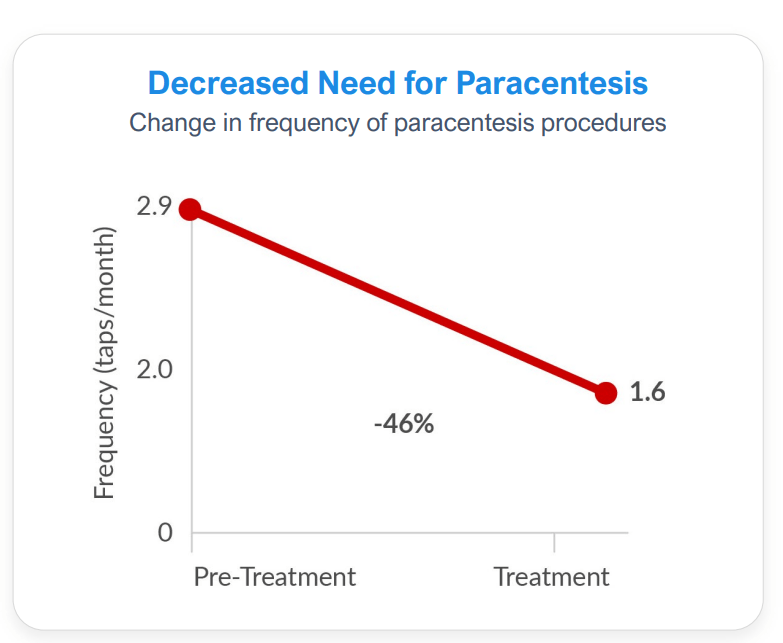
Exhibit 10: Terlipressin Continuous Infusion Efficacy Effects.
The pharmacokinetics (PK) profile of continuous-infusion treatment was compared to that of intermittent IV bolus. Terlipressin plasma concentrations were measured for both treatment mechanisms. Spikes in terlipressin plasma concentration were observed in intermittent IV bolus administration, compared to stable and steady concentration across the time period for continuous infusion. Administration by continuous infusion has proven to be well tolerated with a better safety profile and lower risk of adverse events than with IV bolus.
company presentation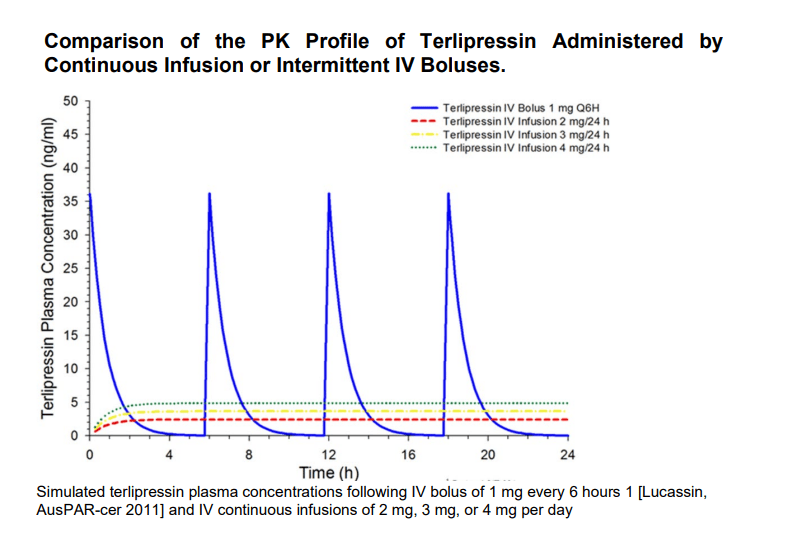
Exhibit 11: IV Bolus v/s Continuous Infusion.
I believe even at this juncture, BIV201 has a considerably higher probability of success, given the pre-established efficacy of the active ingredient, terlipressin. Multiple research papers have been published supporting the case of terlipressin in improving renal function, increasing sodium clearance, and decreasing the need for paracentesis for the treatment of refractory ascites (Bai Z et al. 2020, Krag A. et al. 2007). The recent approval of Terlivaz® (terlipressin-IV bolus) by the U.S. FDA provides further confidence in the drug’s ability to treat life-threatening complications arising from liver cirrhosis, in this case, particularly for hepatorenal syndrome (HRS).
However, the approval did not come easily for Mallinckrodt, as the safety profile included data related to adverse respiratory events in its risk/benefit profile. The new drug application was approved after being resubmitted with a black box safety warning for serious or fatal respiratory failure. A continuous administration of terlipressin is expected to provide an optimal risk/benefit profile, exhibiting similar levels of efficacy with lower risks and adverse events expected, even at lower doses compared to IV bolus. (Cavallin, M. et al. 2015, Lange M. et al. 2007). The European Medicines Agency has recommended that healthcare professionals use continuous infusion over IV bolus in the treatment of HRS.
BIV201 is targeting diseases with high unmet needs via a potentially better administration methodology and pre-established efficacy profile of the active ingredient. The above case for the potential treatment of refractory ascites using BIV201 indicates a de-risked pipeline indication with higher chances of approval. Additionally, the orphan-drug status and fast-track designation provide an expedited development, review, and approval pathway. BIV201 is likely to be administered through a prefilled syringe with a novel liquid formulation, with the fluid being injected into a saline bag attached to a pump.
The drug is currently being evaluated in refractory ascites under a Phase 2b clinical trial with an estimated enrollment of 30 patients. Test results are expected by mid-2023.
Alzheimer’s and Refractory Ascites: Industry and Competitive Overview
NE3107 and BIV201 together represent a multi-billion-dollar market. The company estimates global peak sales potential for Alzheimer’s at greater than $10 billion, while for refractory ascites, it is estimated at over $900 million.
company presentation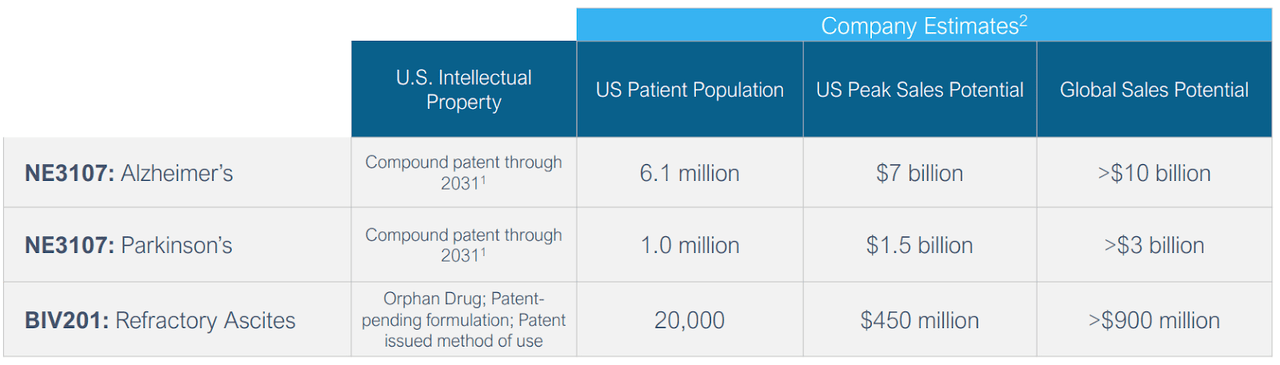
Exhibit 12: Revenue Potential.
Alzheimer’s Disease
Affecting approximately 1.8% of the population, more than 6 million people currently live with AD in the United States. With an aging population, this number is expected to increase steadily, reaching an estimated 13.8 million by 2060. Approximately 50.4% of the patients diagnosed with Alzheimer’s experience mild symptoms, and 30.3% are moderate cases. This indicates a total addressable market of approximately 4.84 million patients. ADUHELM® (aducanumab-avwa), approved in 2021 for mild cognitive impairment or mild dementia arising from AD, has set the cost of treatment at $20,500 for the first year and $28,500 for the maintenance dose in the following years. Assuming NE3107’s cost of treatment falls along similar lines, this represents a total addressable market of approximately $106 billion (4.84 million x $22,000). Based on my estimates and the company’s U.S. peak sales potential of $7 billion, it represents a market share close to 7% based on the current patient population. I believe these are reasonable estimates, even after accounting for multiple competing disease-altering drugs under development in late-stage clinical trials.
|
Company |
Drug |
Current Clinical Phase |
Clinical Result |
|
Cassava Sciences |
Simufilam |
Phase 3 – 1,750 patients |
Phase 2 results: ADAS-Cog11 indicated mean improvement of -1.5 pts. (in 100 patients) |
|
Biogen & Eisai |
Lecanemab |
Phase 3 – 1795 patients (FDA Approved) |
slowed cognitive decline by over 27%, improvement of -0.45 points on CDR-SB scale (phase 3 results) |
|
Annovis |
Posiphen |
Phase 2/3 – 320 patients |
Improvement on ADAS-Cog11 by -4.4 pts (14 patients) |
|
Anavex |
Blarcamesine |
Phase 2b/3 – 509 patients |
Improved cognition on ADAS-Cog by -0.50 points from baseline. (Phase 2b/3) |
|
T3D Therapeutics |
T3D-959 |
Phase 2 – 256 Patients |
Improvement on ADAS-Cog11 by -0.5 pts (early phase 2 results) |
Exhibit 13 Alzheimer’s Disorder Drugs in Late-Stage Clinical Trials (list is not exhaustive)
Simufilam, currently in a late-stage clinical trial, and lecanemab, recently approved by the FDA, have reported optimistic clinical data. Both drugs reported improvements in cognition as measured by the ADAS-Cog11 scale. Lecanemab, which is touted to be a breakthrough in AD treatment, reported clear efficacy on its primary and secondary endpoints. Even with superior efficacy, concerns have been raised regarding the anti-amyloid therapy following multiple deaths in its Phase 3 clinical trial. Safety concerns will be the key factor in lecanemab uptake post-commercialization in 2023, just as they were in Biogen’s previous AD drug, aducanumab. The U.S. Centers for Medicare & Medicaid Services’ (CMS) 2022 coverage restriction for anti-amyloid therapies also limits the drug’s uptake post-commercialization. Inmune Bio’s XPRO1595, another promising disease-altering AD drug that targets the inflammatory biomarker TNF, is also in early-stage clinical trials. XPRO1595 and NE3107 share similarities in their mechanisms to treat AD, with both having neuroinflammation at the core of their focus. Large pharmaceutical companies, including Eli Lilly and Roche, are also evaluating disease-modifying AD drugs in late-stage clinical trials. The growing portfolio of pharmaceutical agents for the treatment of AD in late-stage clinical trials signifies fierce competition in the Alzheimer’s disease space in the coming decade.
BioVie’s NE3107 preliminary data also indicated improvement in cognition along similar lines as observed with simufilam. The direct comparison is certainly not perfect, given the wide differences in patient size, proportions of responders, and other factors, but it certainly provides the gist of NE3107’s competitive positioning.
Refractory Ascites
Ascites and cirrhosis patients account for 116,000 hospital discharges in the U.S. annually, with an observed frequent tendency for early readmissions. Patients requiring paracentesis or the removal of the ascites fluid are admitted for an average of eight days and incur over $86,000 in medical expenses. BioVie believes the TAM for Ascites to be more than $650 million.
Financial Positioning and Valuation
BioVie reported a cash balance of $21.2 million at the end of Q1 2023. For the same quarter, the company’s operating cash burn was $9.22 million, and the average burn in the previous four fiscal quarters was $6.42 million. Additionally, given the recent increase in shares outstanding, it seems that the company has raised funds and is likely to issue more shares going forward, pursuant to its $17.5 million equity raise. Based on the soundness of the science and the positive results from its clinical trials, BioVie will not face any hurdles to raising capital over the short-to-medium term.
|
Total Shares Outstanding |
32.48 million |
|
Current Market Price |
$5.24 |
|
Market Capitalisation |
$170.18 million |
|
Mean Consensus Valuation Estimates / No of Ratings |
$9.75 / 4 |
Source: Seeking Alpha
For a broad outlook, I have valued the company based on its total market opportunity across the three indications in the U.S. Furthermore, I have been conservative with my assumptions regarding the company’s market share and probability of success. The below snapshot explains the calculation yielding an estimated current market value for the company.
Valuation Snapshot, developed for use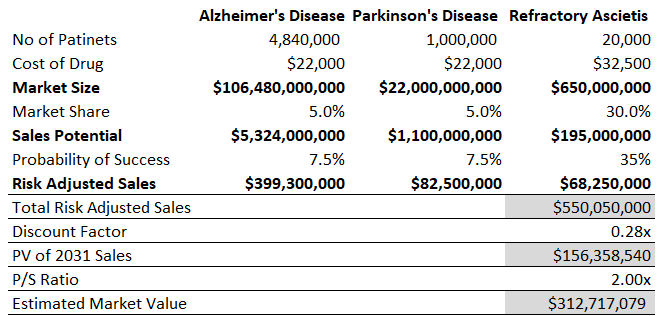
Exhibit 14: Valuation Snapshot
For all three diseases, the sales estimates are indicative of 2031 sales and are discounted for nine years. The above methodology yielded a value of $312.7 million or approximately $9.60 per share. This snapshot is just a brief valuation analysis to get some sense of potential upside.
Risks
-
Capital Raise & Dilution – BioVie’s cash balance, measured in light of its historical and expected cash burn, indicates the need for a cash raise and significant dilution in the coming two-to-three quarters.
-
Competitive Risks – The company’s Alzheimer’s drug NE3107 contributes more than half of its potential peak revenue. The drug faces stiff competition from a growing number of Alzheimer’s drugs in late-stage clinical trials. There are 47 pharmaceutical agents in Phase 3 clinical trials. Big Pharma companies with significant financial resources, like Eli Lilly, Roche, and Biogen, have late-stage drugs under development that are estimated to reach the approval stage by 2024 or 2025.
-
Clinical Trial Risks – Drug development failures and uncertainties are an inherent part of clinical trials, and with complex neurodegenerative diseases like Alzheimer’s, this risk is further amplified. The mean (combined Phases 2 and 3) probability of success for Alzheimer’s is just 2%, compared to 17.8% for all other diseases.
Concluding Remarks
The soundness of the science within both of BioVie’s product candidates is clearly visible, having been explained to the best of my knowledge. The company’s AD and PD candidate, NE3107, has been under extensive research and examination for approximately two decades. It is being developed with strong foundations based on a large body of evidence relating to inflammation and anti-TNF-alpha therapies, which are yielding results as seen in the favorable clinical trial data. BioVie’s primary focus seems to remain NE3107 and the Alzheimer’s disease indication. The recent run-up in the company’s market value is majorly a factor of the robust clinical data reported in its AD biomarker study and the clinical progress in NE3107.
In my opinion, for BIV201, the clinical development risk is considerably low for the obvious reasons stated above, but the risk-adjusted return for BIV201, when compared to that of NE3107, also seems to be comparatively lower.
The strong scientific profile and large market opportunity with multiple catalysts ahead have made BioVie Inc. a high-risk/ high-reward bet with considerable upside potential.


Be the first to comment