
wildpixel
During the after hours of Dec 1, Anavex (NASDAQ:AVXL) announced topline data from their p2b/3 Alzheimer’s Disease, as well as presented the data in the Clinical Trials on Alzheimer’s Disease (CTAD) Congress 2022.
Anavex press release
Following the PR and/or the CTAD presentation, several tweets, e.g. 1st, 2nd (see below), 3rd, 4th, 5th, were critical or skeptical about Anavex’s data.

I’ll discuss some of the critical comments further, as I go through AVXL’s data.
“Changed” Endpoints?
The company said that the trial has met the primary and secondary endpoints with statistically-significant results, while the critics are saying that AVXL has changed the endpoints and therefore any claim of success, i.e. meeting primary and secondary end points, is suspicious.
Fortunately for us, changes made to the protocol can be easily verified here.
As screen shots of the changes are unreadable, please note:
1. Where it says “Reduction in…” (see the table below) used to be “change in” or “changes” before Sept 26, 2021.
2. RSCAQ sleep score was moved from being #3 under Secondary Outcome Measures to #5 under Other Outcome measures (not shown, see here)
The current end points:
|
Primary Outcome Measures :
Secondary Outcome Measures :
|
(complied from clinicaltrials.gov, emphasis added)
Superficially, it would seem that the critics were right in saying that the endpoints were changed, although in my opinion, there is no material difference between using the word “reduction” instead of “change” in describing the cognition data generated from pre-specified measurements, i.e. ADAS-Cog, ADCS-ADL, and CDR-SB.
However, their criticism may be right in a different sense.
I will explain more next.
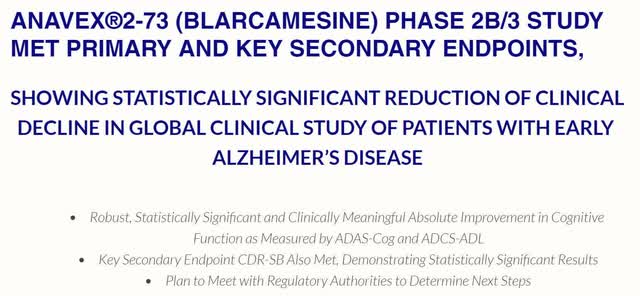
ANAVEX®2-73 (BLARCAMESINE) PHASE 2B/3 STUDY MET PRIMARY AND KEY SECONDARY ENDPOINTS
Besides the all-cap headline above, the announcement’s sub-headlines read:
|
Sounds like a clear-cut win indeed, for a serious, unmet medical need such as Alzheimer’s Disease that has millions of sufferers globally.
As there are two primary endpoints, i.e. ADAS-Cog, ADCS-ADL, and one key secondary endpoint, CDR-SB, one would expect the topline results to show the original mean changes from baseline to 48 weeks in each these three measurements, as well as the corresponding p value for the differences observed between the treatment arms vs. placebo.
Instead, for primary and secondary endpoints, the PR said:
ANAVEX®2-73 demonstrated visible improvement in patients with Alzheimer’s disease. Patients treated with ANAVEX®2-73 were 84% more likely, to have improved cognition by ADAS-Cog score change of -0.50 points or better from baseline to end of treatment than patients on placebo, Odds Ratio = 1.84 (p = 0.015). On average, patients, who improved cognitively with ANAVEX®2-73 treatment, improved by ADAS-Cog cognition score of -4.03 points. ANAVEX®2-73 treatment was 167% more likely to improve function compared with placebo, at a clinically meaningful improvement of ADCS-ADL score change of +3.5 points or better, Odds Ratio = 2.67 (p = 0.0255). This reflects a robust improved and clinically meaningful outcome in cognition and function from baseline. (emphasis added)
Oddly, these results are not the pre-specified endpoint results, as there is no endpoint assessing the probabilities of whether or not responses are more or less likely to be due to the treatment.
Or put it this way: Any trial’s efficacy data is primarily [thus primary endpoint] about investigating possible treatment benefit, and not about investigating the probability of possible treatment benefit.
It is problematic that AVXL is presenting these post-hoc, derivative results as if they are the raw primary data. No wonder that the critics are skeptical.
Regarding the efficacy topline, there’s more in the PR:
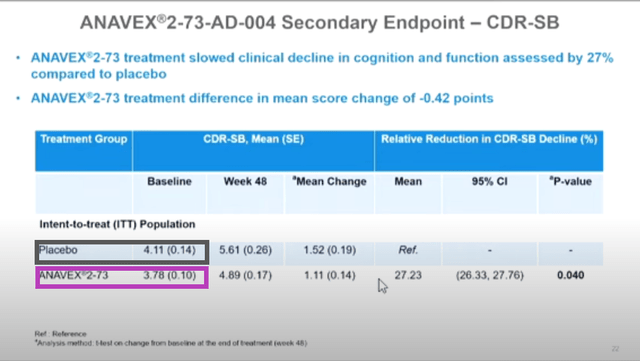
Additionally, treatment with ANAVEX®2-73 statistically significantly reduced cognitive decline, measured with ADAS-Cog, compared to placebo at end of treatment by 45%, representing a treatment difference in mean score change of -1.85 points (p = 0.033).
ANAVEX®2-73 treatment also met the secondary endpoint of reduction in clinical decline of cognition and function assessed by the Clinical Dementia Rating Scale Sum of Boxes (CDR-SB) compared to placebo, by a treatment difference in mean score change of -0.42 points (p = 0.040), representing 27% reduction in the ITT population.
These two paragraphs contain, in my opinion, the real efficacy topline data, which I will discuss more next.
For now, please note two points:
1. The data disclosed in the PR is incomplete, e.g. only the differences in mean score changes are reported*, and not the actual mean score changes in the treatment arms and placebo. Also, it is unclear about the patient numbers of these results, as they are not disclosed.
2. ADCS-ADL data (one of the primary endpoints) is not* reported.
*Note: More results from ADAS-Cog and CDR-SB are reported in the CTAD presentation, but ADCS-ADL data is not reported either in the PR, nor in CTAD presentation.
Problems noted in CTAD presentation
In the evening of Dec 1, besides the PR, the company also put out their CTAD presentation, which was subsequently removed, then later republished with unmarked changes to the original version (which can be seen here), e.g. new footnotes in slides 21 & 22 explaining how the mean change is calculated.
In this section, I’ll address some of the problems noted.
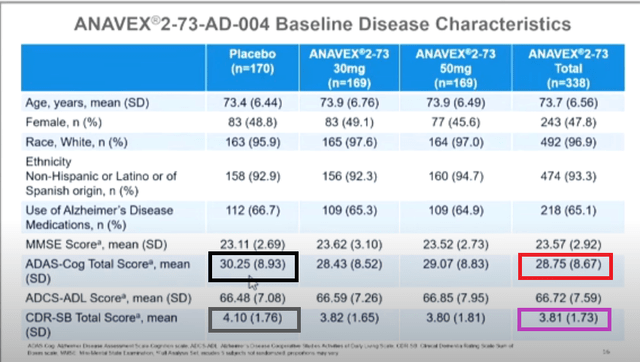
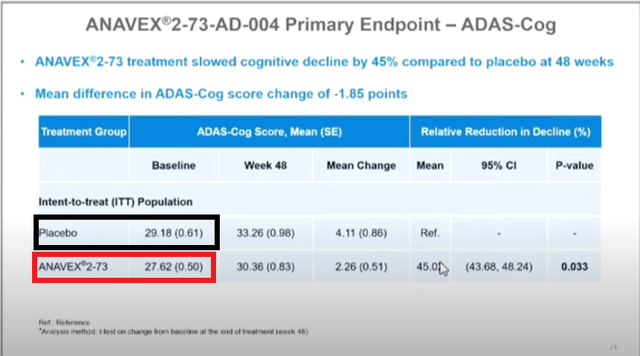
1. “baseline values randomly altered from slide to slide”
Below shows the screen shots of slides 16, 21, 22 from a video of the live CTAD presentation (timestamps 0:25, 1:15, 2:04 respectively), with color boxes added.
CTAD presentation
CTAD presentation
CTAD presentation
(Source)
The data points that are highlighted by the same color box are supposed to represent the same data points. However, as seen, they are not identical.
This discrepancy is either erroneous or they actually represent different data points, e.g. data points in slides 21 & 21 are of different numbers of patients than that of slide 16.
2. “Basic math errors”
One of the tweets pointed out that there seems to be errors in how the mean changes at 48 weeks from the baseline in slide 21 and he put his own calculated results in red numbers.
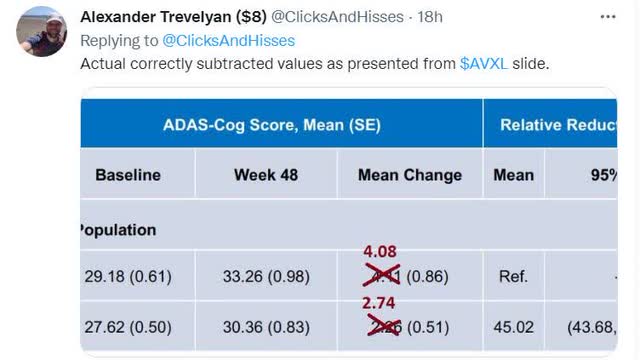
(Source)
So his calculation goes like this:
33.26-29.18=4.08 (instead of 4.11)
30.36-27.62=2.74 (instead of 2.26)
These new mean change would change the difference between the treatment vs placebo to 33% (=[4.08-2.74]/4.08×100%), instead of 45% (p = 0.033) as reported.
One possible explanation I can think of could be that 4.11 & 2.26 are the MEAN of [all the individual] changes, rather than CHANGE in two means, as shown above, which was confirmed by the CEO and new footnotes in slides 21 & 22 from the amended presentation.
3. “Missing Patients’ [Data]”
In AVXL’s CTAD presentation, slides which reported so-called “Efficacy” data, i.e. slide 19 to 22, the patient numbers for placebo and treatment groups are not disclosed.
As for safety data, the table below summarizes the number of “missing patients” whose safety data were not disclosed. No explanation for this data exclusion was given.
| Placebo |
Treatment arms |
|
|
(“Titration phase”) |
170 | 338 |
|
(“Maintenance phase of the trial”) |
161 | 301 |
|
“Missing Patients” |
9=170-161 | 27=338-301 |
(Compiled by author)
Discussion
It is unfortunate that the reported results, either in the PR or in CTAD presentation, have problems and are incomplete, even for topline data.
Firstly, as I noted earlier, some of what AVXL presented in the PR as “positive” topline results are in fact a post-hoc analysis, and not the actual topline efficacy data.
Secondly, unlike the ADAS-Cog and CDR-SB data, no ADCS-ADL data was reported, either incompletely in the PR, or the toplines in the CTAD presentation.
Since ADCS-ADL is one of the two primary endpoints, which AVXL claims to have been met with statistically significant data. Its absence is troubling. Does the company expect the public simply to believe in faith that ADCS-ADL data is good, without ever seeing the data?
Thirdly, the data presented is pooled data from two dose arms (30mg, 50mg), which the company claims that they have not yet “run through”. Will either or both of them, when “run through”, still show a statistically-significant treatment effect over placebo, as for the combined data, e.g. a p value of 0.033 in ADAS-Cog, unknown in ADCS-ADL, 0.04 in CDR-SB?
Fourthly, there is no actual data from “patients without SIGMAR1 gene mutation” to know how this “provides further confidence of the robustness of the SIGMAR1 activation in the treatment of neurodegenerative diseases”.
Lastly, the “Missing Patients'” safety data.
Conclusion
Developing drugs to meet an unmet medical need is a costly, lengthy, difficult process that require patient endurance of all stakeholders.
I commend the company and the investors for their dedication to this meaningful and important endeavor. I hope that AVXL team is not discouraged by the close scrutiny paid thus far to the topline data of their AD p2b/3 trial. If some of the criticism seems harsh, it’s only because big claims demand big evidence. I wish them all the best in continuing their endeavor to develop 2-73 to be a safe and effective treatment in early Alzheimer’s Disease and in other programs.
For the rating, I’d choose HOLD, or neutral, as I think AVXL can be a worthy long-term investment opportunity for those who have the right risk tolerance and time frame and are interested in the neurodegenerative diseases space.
The readout from their p2/3 EXCELLENCE trial in pediatric patients with Rett syndrome is expected in H1 2023 as well as the open-label extension trial in Parkinson Disease Dementia by YE 2022 or Q1 2023.
Thanks for reading. Hope this article is clear and helpful in your research on AVXL. All the best!


Be the first to comment