
Krittiraj Adchasai/iStock via Getty Images
Introduction
On Wednesday March 30, after a whole day of online meeting that included very moving testimonies from amyotrophic lateral sclerosis (ALS) patients, FDA’s advisory committee voted 6 No to 4 Yes, to the question of whether Amylyx’s (NASDAQ:AMLX) single phase 2 trial data established its effectiveness in treating ALS.
Prior to the meeting, as noted by AMLX’s March 28 PR, the FDA had released the briefing documents which include presentations/positions from both the company and the FDA.
Perhaps evidenced in the volatile price action of the stock over the past 5 days (AMLX’s stock was halted on March 30), the overall market reaction is negative due to the uncertainty of AMLX’s ALS drug candidate’s, AMX0035, approval prospects.
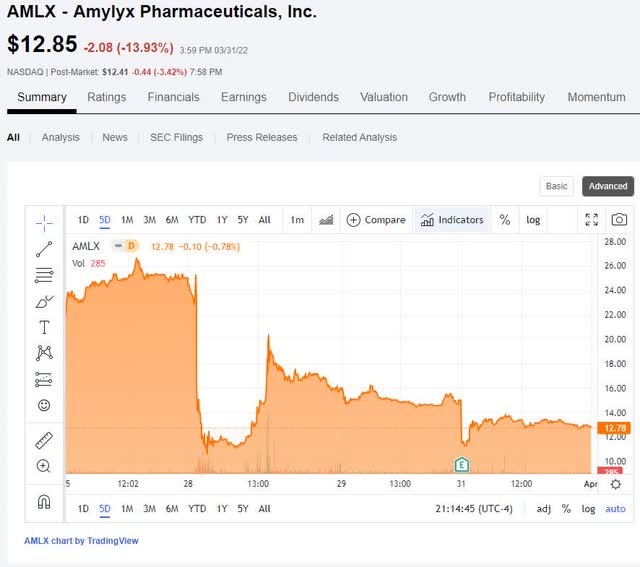
seekingalpha.com
(AMLX 5-day chart ending on March 31)
In spite of the negative vote (admittedly a narrow one), “[the company] remain confident in the data from the Phase 2 CENTAUR trial and the potential benefits of AMX0035 as a treatment option for people living with ALS,” said Jamie Timmons, M.D., Head of Scientific Communications of Amylyx in a March 30 PR.
From the same PR, AMLX states:
Although the FDA considers the recommendations of its advisory committees, the recommendations by the panel are non-binding. The final decision regarding approval of a pending NDA is made by the FDA. As previously reported, the FDA has granted Priority Review to the NDA for AMX0035 and is expected to make a decision on the approval of AMX0035 by June 29, 2022 under the Prescription Drug User Fee Act.
(Source; Emphasis added)
AMX0035’s Efficacy data: AMLX vs. FDA
It is important to know that there’s no disagreement between the FDA & AMLX regarding the urgent need for more (safe and effective) ALS treatments; or regarding the safety profile of AMX0035.
However, it’s clear that there are two perspectives regarding the efficacy data.
The briefing documents and the advisory committee meeting seem, to me, to not have changed the positions of either side (see the Table below).
| Benefit-Risk Summary |
|
The Applicant’s [AMLX] Position: […] Analyses of AMX0035’s efficacy over 24 weeks demonstrated clinically meaningful slowing of ALS (function, strength and breathing) as well as statistically significant benefits in ALSFRS-R compared with placebo (both combined with standard of care). Results were consistent across multiple outcomes and analyses. Furthermore, the benefit of AMX0035 was maintained during a 48-week analysis of ALSFRS-R and additional secondary outcomes. Additionally, overall survival, ventilation, and hospitalization were assessed over long-term follow-up and demonstrated significant improvement in participants treated with AMX0035. […] AMX0035 is the first therapeutic to show a benefit on both survival and function in ALS. In the context of a rare, rapidly progressing and life-threatening disease with high unmet medical need, the efficacy and safety demonstrated in CENTAUR support approval of AMX0035 for the treatment of ALS. (Source: page 96; abridged for brevity, emphasis added) |
|
The FDA’s Position: […]
(Source: page 97; abridged for brevity, emphasis added) |
*Note: Throughout the briefing documents, the FDA stated their concerns and provided their own statistical analysis.
The table below gives two examples of different p values given by ALMX & FDA regarding the primary and one of the secondary endpoint, which is to do with the treatment benefit on function.
|
AMLX |
FDA | |
| Primary endpoint (ALSFRS-R rate of decline), page 45 & 47 | p = 0.034 | p = 0.1134 |
| One of the secondary endpoint (Upper ATLIS), page 58 & 59 | p = 0.042 | p = 0.2319 |
In summary: Although AMLX claims that “AMX0035 is the first therapeutic to show a benefit on both survival and function in ALS”, the FDA does not seem to think that the effectiveness of AMLX’s ALS drug is conclusively established by their p2 trial data.
ALS treatment landscape
Before I conclude, I would like to briefly describe the ALS treatment landscape.
Firstly, there are five FDA-approved drugs for treating ALS symptoms, according to ALS association, including Mitsubishi Tanabe’s Radicava™(IV edaravone), which was approved in 2017.
Though the exact mechanism of action is unknown, this 2018 paper thinks that edaravone’s treatment benefit may be due to “its known antioxidant properties”.
In the FDA approval label (see page 8), it’s noted that the primary end point (ALSFRS-R total scores change from baseline to week 24) was met with a much more conclusive statistical significance (p=0.0013) than AMLX’s p=0.034.
It’s also worth noting that Mitsubishi Tanabe’s oral formulation of Radicava for ALS has its PDUFA date on May 12, 2022.
Secondly, there are several late stages ALS trials ongoing currently (see below).
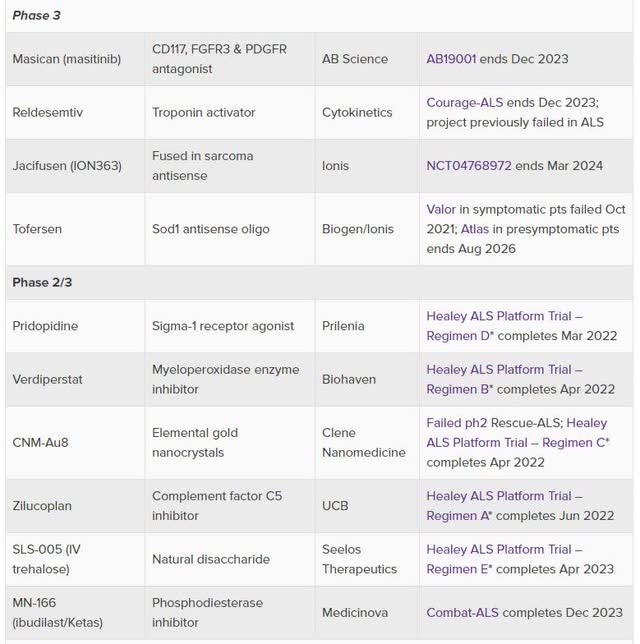
Evaluate.com
(Source)
Two notables for me:
(1). Prilenia’s Pridopidine, a Sigma-1 receptor agonist, would share a similar mechanism of action with AVXL’s drug candidates, also Sigma-1 receptor agonists, which have shown promising results in serious neurodegenerative diseases, e.g. PD, Adult Rett Syndrome, AD etc.
According to Prilenia:
Pridopidine one of the first 4 drug candidates being studied in the Healey ALS platform trial. It was selected from an international competition of >30 potential therapeutics by an independent expert review committee based on compelling human genetic validation data, efficacy in preclinical models and favorable safety profile.
(2) Healey ALS Platform Trial, sponsored by Massachusetts General Hospital, has been studying multiple ALS treatments since July 2020 (n = 800) and the estimated primary completion date is April 2023.
[Healey ALS] platform trial is designed to accelerate development of promising new treatments by enabling investigators to simultaneously assess multiple therapeutic candidates, enabling pooling of placebo data across regimens (which minimizes the number of patients on placebo).
(Source)
Lastly, a brain-computer interface implant “deemed safe after one year in ALS patients” seems to be helping the first two ALS patients “perform daily tasks like sending text messages and emails, as well as shop and bank online.”
Of course, there is no certainty that any of these drugs or device will be successfully developed.
However, the fact that there are approved drugs which may work to slow the progression, and several late-stage trials on-going that expect readouts in 2023 should be a reason to be hopeful.
Concluding Thoughts
For investment consideration, I think that it’s a gamble, as there is no way to estimate the likelihood of AMX0035’s chance of an approval, full or accelerated.
On the strength of the p2 trial data, I agree with what was expressed in the FDA’s positions in the briefing documents and the Advisory committee’s 6-4 vote that the effectiveness of AMX0035 is not conclusively established.
However, the FDA may indeed use its “regulatory flexibility” on the basis of an urgent, serious unmet medical need, i.e. more ALS treatment options.
In a scenario where the odds are not possible to estimate, I think it’s prudent to risk only little for those who wish to do so.
Thanks for reading.


Be the first to comment