Phira Phonruewiangphing/iStock via Getty Images Albireo

The successful investor is usually an individual who is inherently interested in business problems. – Phillip Fisher (Warren Buffett’s one of two mentors)
Author’s Note: This article is an abridged version of an article originally published for members of the Integrated BioSci Investing marketplace on October 11, 2022.
In biotech investing, there are two themes that you should focus on. The first is whether an approved drug can generate sales traction. The second is if the company continues to expand the approved product’s label. With more label expansion, you can expect to see leaping sales growth. Ultimately, that would translate into a higher share price in the long term.
That being said, I’d like to revisit Albireo Pharma, Inc. (NASDAQ:ALBO). With its lead medicine (Bylvay) already approved for a liver condition coined PFIC, Albireo is expanding its label for two additional indications. With the recent positive data release for the second label, you can bet that the fundamentals of Albireo are substantially strengthened. In this research, I’ll present a fundamental analysis of Albireo and share with you my expectations for this intriguing equity.
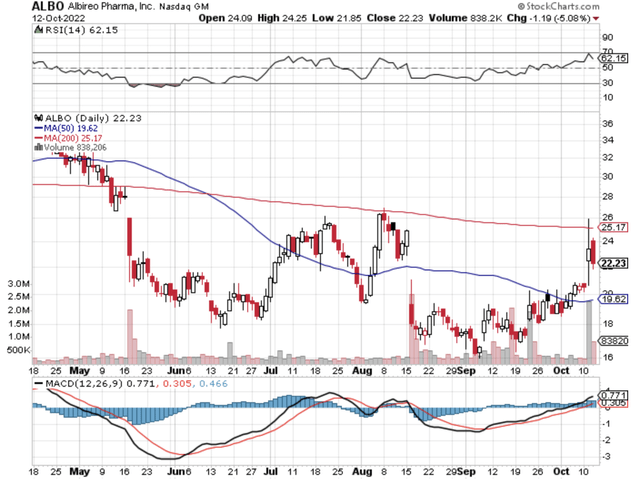
Figure 1: Albireo Pharma chart
About The Company
As usual, I’ll provide a brief overview of the company for new investors. If you are familiar with the firm, I recommend that you skip to the subsequent section. As noted in the prior research:
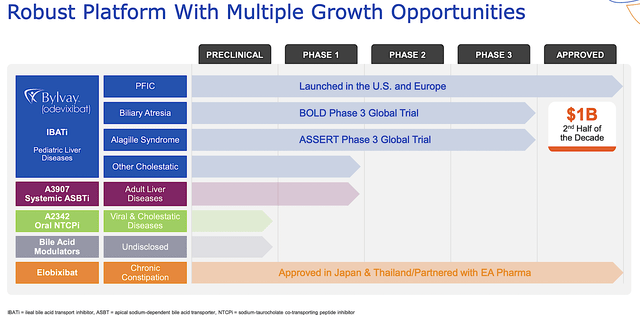
Operating out of Boston, Massachusetts, Albireo Pharma is focused on the innovation and commercialization of novel medicines to fill the unmet needs in orphan liver conditions. As shown below, the company has an interesting portfolio of two approved drugs and other early-stage developing therapeutics (i.e., A3907 and A2342). As the crown jewel of this pipeline, odevixibat (Bylvay) is authorized to treat the rare genetic liver condition, progressive familial intrahepatic cholestasis (i.e., PFIC) that is characterized by extreme itching. The other notable molecule is elobixibat, approved for the treatment of chronic constipation (of which Albireo receives royalty payment from EA Pharma).
Figure 2: Therapeutic pipeline
Mechanism Of Action: Bylvay’s
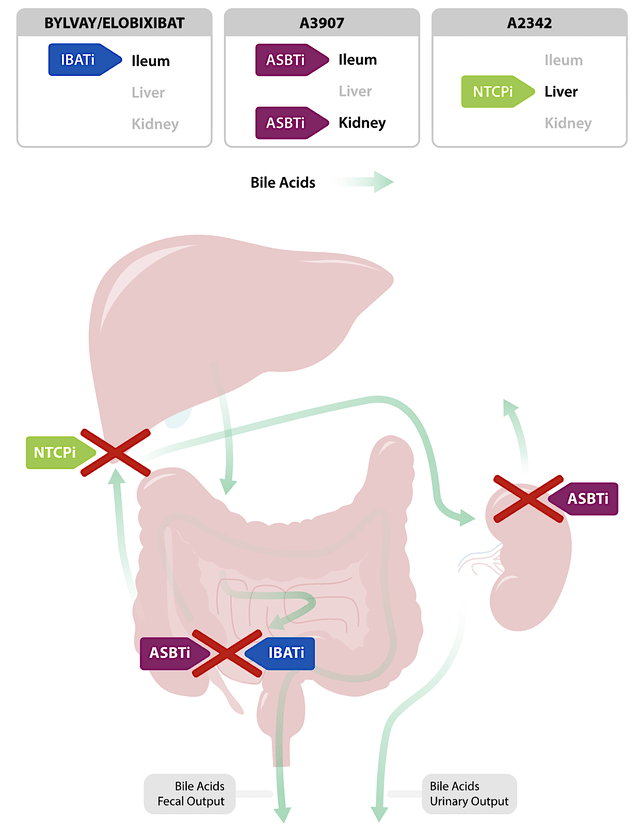
As you can appreciate, Albireo’s success rests on Bylvay’s backbone. As such, you should go over the important details of this drug. As a potent, once-daily, non-systemic ileal bile acid transport inhibitor (i.e., IBATi), Bylvay works by blocking the bile acid reuptake in part of the small intestine coined ileum. As such, it reduces the overwhelming level of bile acid that causes devastation in patients suffering from conditions having bile acid overloads: PFIC, Allagile Syndrome, and biliary atresia.
Notably, you can see that the “mechanisms of action” (i.e., MOA) of Bylvay (bile acid reduction) would work great for diseases that resulted in bile acid overloads. Here, the puzzle pieces — “MOA and disease context” — fit together beautifully.
Figure 3: Bylvay mechanism of action
As you can appreciate, Bylvay is already generating increasing sales for its first approved indication, PFIC. Specifically, Bylvay is approved to treat the pruritus (i.e., itching) associated with PFIC. In the USA, it is authorized for use in patients 3 months and older who suffer from progressive PFIC.
In the EU, it is launched in patients at least 6 months or older in all types of PFIC. Interestingly, Bylvay also has two pending label expansions. The first (and most advanced) expansion is for Alagille. The second expansion is for biliary atresia.
Figure 4: Bylvay indications
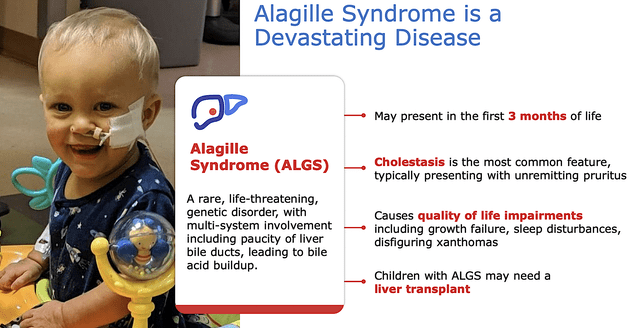
Disease Context: Alagille Syndrome
To see how the disease context of Alagille Syndrome harmonizes with Bylvay’s MOA, you should go over the generalities of Alagille Syndrome. As a rare and multisystem genetic disorder, Alagille affects 25K people worldwide.
The main feature of Alagille is extreme itching due to bile acid elevation secondary to duct blockage. In other words, the blockage prevents the release of bile acid from the liver to the small intestine for excretion. Hence, the level of bile acid builds up in the blood. Consequently, this affects many systems in the body such as the heart, skeleton, eyes, brain/spinal cord, kidneys, and face.
Coming back to what we discussed about the MOA of Bylvay, you can see how a drug like Bylvay that ultimately reduces bile acid would be a great fit for Alagille. Simply put, the MOA and disease context goes well here, which explicates the robust data release for upcoming label expansion that I’m about to discuss.
Figure 5: Alagille Syndrome
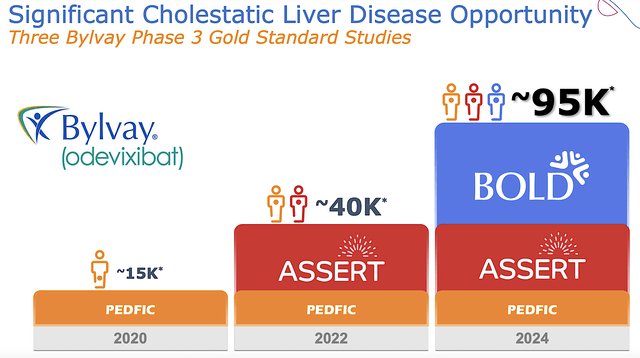
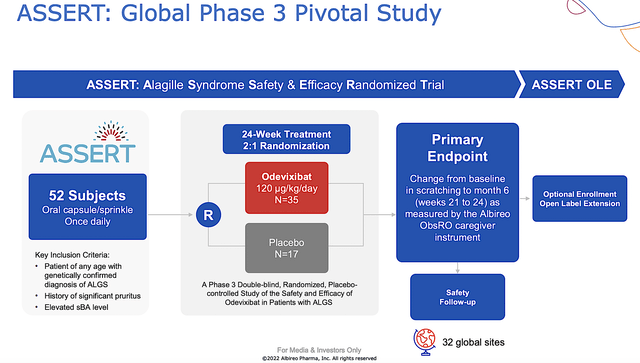
Supporting Data: Robust ASSERT Results
Shifting gears, the good news came on October 11, when Albireo published the robust topline results for the Phase 3 (ASSERT) trial. As a high-quality global double-blind, randomized, placebo-controlled trial, ASSERT assessed the efficacy and safety of Bylvay for Alagille syndrome in patients from birth to early adulthood.
Figure 6: ASSERT study design
As shown in the figure below, ASSERT cleared both its primary endpoint of improvement in pruritus and its key secondary endpoint of reduction in serum bile acids with flying color. Given that the p-values were less than 0.05, you can bet that the finding is real rather than by random occurrence.
Figure 7: Strong ASSERT findings
Safety-wise, Bylvay was very well tolerated with the rate of diarrhea related to the drug being minimal. Another metric that you can use to gauge the safety of the drug is the study discontinuation rate. Since there was no discontinuation, you can tell that the safety profile is excellent. According to the Pediatric Gastroenterologist and Hepatologist (Dr. Nadia Ovchinsky) who is the lead investigator:
The robust results from the ASSERT Phase 3 trial are important because physicians urgently need more options in the treatment of Alagille syndrome. Bylvay reduced the devastating pruritus, which is so common among this patient population and critical to helping us improve sleep, among other burdens of the disease. The study also showed that Bylvay reduced serum bile acid levels, which could indicate that Bylvay may diminish the severity of liver disease over time, an important consideration for me as a treating physician.
Toward Approval
Riding the stellar data, Albireo is already in discussion with both the FDA and EMA about regulatory filing for approval. However, the sample of the study was a bit small with only 52 patients. Moreover, this is only one single study. Therefore, you might question whether ASSERT findings would be adequate to gain approval.
Taking all the aforementioned puzzle pieces into consideration, I strongly believe the answer is yes. After all, Alagille is a rare condition. As such, it’s difficult to recruit more patients for the trial. Secondly, the trial design with randomization, and placebo-controlled are high-quality designs. There is also a strong demand for novel treatments for Alagille and Bylvay is the answer. Simply put, the MOA of Bylvay, the disease context of Alagille, and the strong data support approval.
Wasting no time, Albireo is poised to immediately submit a filing for approval with both the FDA/EMA. Commenting on recent developments, the President and CEO (Ron Cooper) remarked,
Our ASSERT Phase 3 study demonstrated early, rapid, and sustained effects on reducing pruritus and bile acids in Alagille syndrome, as it did in the Phase 3 PFIC study. We have successfully completed two of three gold-standard pediatric liver disease studies, look forward to imminent full enrollment of a third Phase 3 with our BOLD biliary atresia study, and with more than $270M in cash, we have sufficient resources to execute our plans. At the same time, we are seeing an acceleration in PFIC revenue and expect Q3 Bylvay sales to be above $7M.
Financial Assessment
Just as you would get an annual physical for your well-being, it’s important to check the financial health of your stock. For instance, your health is affected by “blood flow” as your stock’s viability is dependent on the “cash flow.” With that in mind, I’ll analyze the 2Q2022 earnings report for the period that ended on June 30.
As I already went into great detail about the financials in the previous research, I’ll briefly mention the pertinent findings here. Accordingly, Albireo procured $8.2M in revenues compared to $2.4M for the same period a year prior. That aside, the research & development (R&D) for the respective periods registered at $22.8M and $20.8M.
Moreover, there were a $39.9M ($2.04 per share) net losses compared to a $36.4M ($1.90 per share) decline for the same comparison. Moreover, the company had $181.0M in cash and equivalents which should allow operations into 2024 before the need for additional financing.
Potential Risks
Since investment research is an imperfect science, there are always risks associated with your stock regardless of its fundamental strengths. More importantly, the risks are “growth-cycle dependent.” At this point in its life cycle, the main concern for Albireo is whether the company can continue to ramp up Bylvay sales. It’s difficult for a small company “going at it alone” in commercialization to see a quick sales ramp-up.
That aside, there is a 35% chance that BOLD would generate negative data in 2024. In case of failed data, I believe that the stock would tumble but not as aggressively as 50% because Bylvay already secured one approval.
Final Remarks
In all, I recommend Albireo Pharma as a speculative buy with a 4.8/5 stars rating. Albireo is a story of investment and innovation success. Harnessing the therapeutic power of Bylvay, Albireo is already enjoying commercial success with the PFIC launch. With the robust ASSERT data, it is assertive to say that Bylvay would be approved for Allagile. Though being an orphan condition, there is a premium reimbursement to reward the innovation process. Now, I would be bold to forecast that the other Phase 3 study for biliary atresia would also deliver excellent outcomes.



Be the first to comment