da-kuk/E+ via Getty Images
People are judging TG Therapeutics (NASDAQ:TGTX) on Ukoniq’s poor show in CLL. I think ublituximab’s data in Multiple Sclerosis is the basic investment thesis in TGTX. I could be wrong, but I will present both the bull and the bear case for TGTX here today, given the disaster that happened on Friday.
Ublituximab’s MS positioning
Where does ublituximab position itself among the choices available in MS currently? For doctors, it sort of depends on the patients. If a patient is able to take the infusion herself, then there could be self-infusible drugs like Kesimpta. If the patient cannot, she can go to an infusion center. If she is afraid of needles, she can take an oral drug. If she is okay spending half a day every six month at an infusion center, and she wants an old and proven drug, then it’s ocrevus for her. If she would prefer one hour infusion over 4 hours, and she is okay with new and proven, then it can be ublituximab. This is how Professor Lawrence Steinman positions ublituximab in the MS “armamentarium.”
Professor Steinman is the Global study chair for ublituximab in MS and the Zimmerman Professor of Neurology and Neurological Sciences, and Pediatrics, Stanford University. I like his conservative summary, but you should read between the lines as well. For an established scientist like Professor Steinman to put a “new and proven” drug like ublituximab ahead of an “old and proven” drug like ocrevus would be risky, especially when he is paid by TGTX. However, note how he uses words like “spectacular” and “delightful data” when he describes the below 10% ARR (Annualized Relapse Rate) achieved by ublituximab, which he says no other MS drug has achieved before. Recall the other small and not-so-small advantages of ublituximab over ocrevus we have discussed before, and will do so here again, and you will realize it all comes down to “new and proven” versus “old and proven” and the matter of convenience and insurance coverage. To overcome that difference is not a scientific pursuit any longer, but a marketing one. By that I mean, I believe ublituximab will be approved, and if ocrevus patent doesn’t expire soon (earliest date is 2029), then, if TGTX management can sell it well, it can become a blockbuster. You need to completely separate ublituximab with umbralisib in your mind in order to assess TGTX fairly.
Infusion duration
Focus on this as a patient. Today you are going to the infusion center for your MS medicine. You are taking OCR so you will need 4-5 hours of infusion. If you were taking UB, you could be out in 1-1.5 hours, avoid all those germs, because infection is the big problem with MS therapy, right? If OCR is way better than UB, you wouldn’t mind. But it isn’t; data here says so. On many aspects, UB is superior to OCR. In some aspects, it is comparable. If the company can sell this point to a patient, they would opt for the 1 hour infusion.
From the point of view of the infusion center, as well, that’s 4-5 times more patients per day. If you ignore the critics and consider it carefully, then, ceteris paribus, infusion duration becomes a really important differentiator.
Competitive differences
Critics of TGTX harp about the differentiated MS market, with more than 15 drugs in the market vying for attention. What they forget to tell you is that ALL 15 DRUGS ARE FROM BIG PHARMA. Ublituximab is the ONLY drug from a small company. So the upside can be high.
They also do not tell you that the MS market may be diverse, but it is not saturated. There’s still a lot of room for improvement, some of which could be almost “aesthetic,” like less infusion site reaction, shorter duration of infusion, as well as lower ARR rate, competitive disability progression/improvement scores, high reduction in lesions, and so on – in all of which ublituximab has shown a competitive profile.
It is also a fact that while there are many MS drugs, there are only 2 approved CD20 drugs – ocrelizumab (OCR) and ofatumumab (OFA). Ritixumab is also used off-label. That makes 3 anti-CD20 drugs.
CLL withdrawal
On Friday, TGTX announced they are voluntarily withdrawing their BLA for U2 in CLL after latest data “showed an increasing imbalance in OS.” I have discussed this before, so I will not discuss this here again. All I will say is that CLL isn’t dead, but if it ever revives, that will take considerable time and effort. I will also say that this Monday TGTX stock will be devastated; which could be an opportunity.
Rather, I will focus on exploring ublituximab in MS from as many angles as I can find.
How does ublituximab work – comparative mechanism of action discussion
Ublituximab is an anti-CD20 antibody, but it is also glycoengineered, meaning certain sugar molecule positions on the antibody have been altered to increase the potency of the molecule. This is called glycosylation. Existing anti CD20 MS antibodies like ocrevus, ublituximab’s chief competition, are not glycoengineered. This process allows “cytokines released from the B cells to be metabolized within phagocytes rather than to be released into the bloodstream.”
Anti CD20 antibodies target the cell surface CD20 receptor present on all B-cells, but most efficiently the circulating B-cells. There are two killing mechanisms here, antibody-dependent cellular cytotoxicity (ADCC), and complement-dependent cytotoxicity (CDC). In ADCC, the antibody signals immune cells like NK cells to kill the B-cell. In CDC, the complement cascade, a group of immunological proteins, is activated. The complement system has a major role in autoimmune processes, so activating it can have side effects.
Ublituximab has low fucose content so it can have enhanced ADCC, helped by the molecule’s strong affinity to FcγRIIIa receptor protein. This causes high numbers of NK cells to do ADCC regardless of CD20 expression numbers. Among all CD20 antibodies, ublituximab possesses the highest efficacy regarding ADCC. It also has a decidedly lower CDC, which makes it safer.
Comparative efficacy profile
Like I said, ocrevus is going to be UB’s chief competitor. In the 2 phase 3 trials, however, aubagio or teriflunomide was used as comparator, because, the company explained, teriflunomide is standardly used in registrational MS trials.
Our job is to compare UB with ocrevus through a cross trial comparison. I discussed all that earlier; but given the current state of fear, here’s it once again.
Ublituximab Ocrevus comparison (TGTX website)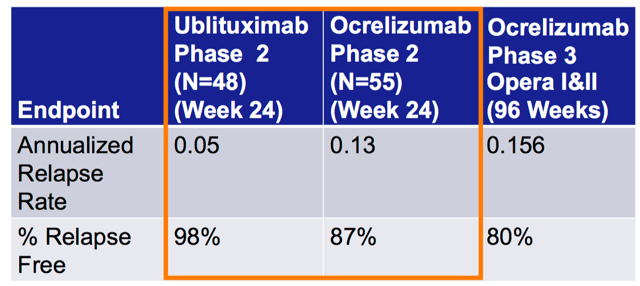
Now that we have the phase 3 data also, we can add one more column here, which will show that the phase 3 ARR was 0.07; still below 0.1, which means a single relapse every ten years. Ocrevus’ data would imply a single relapse every 7 years, while UB’s would be once every 14 years. That’s a major difference. Ask any RRMS patient.
Disability progression versus disability improvement
This was a secondary endpoint of the ULTIMATE trials (note, the two trials have no difference in design). There are two metrics here, confirmed disability improvement or CDI and confirmed disability progression or CDP. One is a measure of how much the disability improved, while the other is a measure of how much the disability worsened. The data was:
At 12 weeks, 5.2% and 5.9% of ublituximab- and teriflunomide-treated patients, respectively, had confirmed disability progression (CDP), while only 3.3% of ublituximab-treated patients showed a 24-week CDP, compared to 4.8% of patients in the teriflunomide group. Neither was deemed statistically different.
So the trial failed this secondary endpoint, so this could be one of the rare things a critic could talk about. However, University of California San Francisco neurologist Bruce Cree, MD, PhD, and an ULTIMATE investigator, explains this in an interview. He says that CDP and CDP are measured using the Expanded Disability Status Scale (EDSS). The EDSS scale, he says (and I have confirmed independently from other research), is a challenging score because it can change very little over the course of a trial.
“Because confirmed disability worsening was not met as one of the secondary endpoints, one of the critiques of these trials could be that there wasn’t an effect of ublituximab. But worsening disability was rare in both treatment arms, so it would be very difficult, if not impossible to demonstrate a difference without much greater numbers of patients being included,” he said.
The multiple sclerosis functional composite (MSFC) score, Cree says, is another more sensitive score. It measures disability using three different tests: the 9-hole peg test, which assesses upper arm mobility; the timed 25-foot walk test, which gauges walking ability; and the paced auditory serial addition test (PASAT), a measure of attention and processing.
Data from the 2 trials showed that ublituximab significantly improved the MSFC score by 76% and 89%, compared with teriflunomide. Here’s the data in a table:
|
UB |
Teriflunomide |
p-value |
|
|
ULTIMATE 1 |
0.469 |
0.266 |
0.048 |
|
ULTIMATE 2 |
0.521 |
0.275 |
0.017 |
Source- author
Moreover, there’s data that shows improvement in CDI:
A pre-specified pooled tertiary analysis looking at confirmed disability improvement (CDI) showed that 12% of those treated with ublituximab had 12-week CDI compared to 6% of those in the teriflunomide group, representing a 116% increased chance of improvement (P = .0003). Similarly, 9.6% and 5.1% of those in the respective groups had 24-week CDI, equating to a 103% increased chance (P = .0026).
I think that satisfactorily addresses the failure in this secondary endpoint. Also note that many approved MS therapies haven’t passed all secondary endpoints.
Safety profile
All the anti CD20 antibodies are generally well-tolerated by MS patients, with some variations. For example, for ocrevus, here’s some interesting data for infusion-related reaction (IRR):
In the OPERA studies, most of the IRRs were mild to moderate, but one OCR receiving patient experienced a life-threatening episode of bronchospasm during the first infusion and was withdrawn from the trial [29]. Regarding ORATORIO, no fatal or life-threatening IRR occurred. Nevertheless, two patients in the OCR (0.4%) withdrew from the treatment due to IRRs [42].
So that’s a major safety issue for ocrevus – if it had happened with ublituximab, TGTX bears would surely have given it a lot of focus.
Coming to the ULTIMATE trials, here’s the data:
Both treatment groups had similar number of adverse events (AES), the most common being infusion-related reaction (IRR), headache, nasopharyngitis, and lymphopenia. No patients treated with teriflunomide had grade 4 severity of IRRs compared to 0.2% of those in the ublituximab group at weeks 1 and 3. IRRs were most frequent on the first dose, with 43% of those in the ublituximab group and 9.7% of those in the teriflunomide group reporting IRR on day 1. Most IRRs were mild to moderate and decreased in frequency with subsequent dosing.
Serious AEs (SAEs) occurred in 6.2% and 9.5% of the teriflunomide and ublituximab groups, respectively. The most common SAEs, occurring in at least 1% of any treatment group, was infections and infestations, as well as nervous system disorders.
Three deaths occurred in the trial, resulting from encephalitis, salpingitis, and pneumonia, all in the ublituximab group. Investigators deemed that the case of pneumonia was possibly related to treatment. Additionally, investigators saw no cases of progressive multifocal leukoencephalopathy.
According to Fox et al. [61], higher doses and faster infusion times do not correlate with higher rates of IRRs for UB. Moreover, although quite similar, UB has a slight edge in IRR.
The FDA has approved a shorter 2-hour infusion time for ocrelizumab in December 2020, but there’s a caveat. This approval is only for patients who have not experienced any prior serious infusion reactions. I believe that’s a very small number given how many patients experience some IRS.
Coming to the pneumonia death, it is a measure of how well a B-cell killer works that infections occur. Thus, the death is more a comment on patient management in the trial than with how well UB worked – the patient died because it worked very well and depleted his B-cells.
One issue I could not directly resolve is immunogenicity. Since both OFA and OCR are humanized to an extent, they are supposed to cause less immunogenicity than the chimeric UB. However, trial data for this measure is not available for UB, except in this first-in-human trial where no cases of serum anti-UB antibodies were detected.
Bottomline
Today, TGTX has a market cap of $1.26bn and a price of $8.85. On Monday, the stock may fall 30% or more – it all depends on how little the market understands TGTX.
My cost basis is about double today’s price. If TGTX falls 30% on Monday, it will be priced at around $6.
On September 28, TGTX has the MS PDUFA. I have studied TGTX in MS from every possible angle. The CEO has a habit of announcing bad news like a rabbit out of the hat. Last time it was in a fireside chat, this time it was before a long holiday weekend. So I can’t comment if he has some bad news on MS up his sleeve. But that’s not a scientific or rational discussion.
From every science angle I have seen, ublituximab’s LoA or likelihood of approval is absolute. I can’t find a chink in the armor. Granted, its success in the market is a different ballgame, but I don’t think the critics are right when they say the chances of commercial success is low. I have just discussed why.
Given all of that, I am seriously debating doubling down at the lowest price possible on Monday, and waiting for September 28.


Be the first to comment