asbe/iStock via Getty Images
On December 13, 2022, Moderna, Inc. (NASDAQ:MRNA), a large ($63 billion market cap) biotechnology company pioneering messenger RNA (“mRNA”) therapeutics and vaccines, and Merck (MRK) announced that a Phase 2b trial of an investigational personalized mRNA cancer vaccine (“PCV”) demonstrated a “statistically significant” and clinically meaningful improvement in the primary endpoint (“PEP”) of recurrence-free survival (“RFS”) versus placebo for the adjuvant treatment of patients with stage III/IV melanoma following complete resection. The market was very receptive to the announcement, and long-term investors can look forward to more positive catalysts arising from the PCV’s continued development.
Background info in the press release [with commentary]
-
Melanoma is the most serious form of skin cancer, with an expected 99,780 new cases of melanoma diagnosed and 7,650 deaths in the U.S. in 2022. Moderna cites 5-year survival rates estimated to be 60.3% for stage III and 16.2% for stage IV.
-
KEYNOTE-942 is a randomized, open-label Phase 2b trial that enrolled 157 patients. Following complete surgical resection, patients were randomized to receive mRNA-4157/V940 (nine total doses of mRNA-4157) and KEYTRUDA (200 mg every three weeks up to 18 cycles) versus KEYTRUDA alone for approximately one year until disease recurrence or unacceptable toxicity. Secondary endpoints include distant metastasis-free survival and safety. [According to National Comprehensive Cancer Network (NCCN) guidelines, KEYTRUDA is a category 1 option (has high level of evidence) for patients with stage III with resectable sentinel lymph node (“SLN”) metastasis, as well as for unresectable disease.]
-
Key eligibility criteria for the trial included:
-
resectable cutaneous melanoma metastatic to a lymph node and at high risk of recurrence [high risk is based on the maximum SLN tumor diameter being >1 mm, according to the Rotterdam Criteria],
-
complete resection within 13 weeks prior to the first dose of KEYTRUDA,
-
disease free at study entry (after surgery) with no loco-regional relapse or distant metastasis and no clinical evidence of brain metastases,
-
tumor sample available suitable for sequencing,
-
Eastern Cooperative Oncology Group Performance Status 0 or 1 [fully active; or restricted in physically strenuous activity but ambulatory]
-
normal organ and marrow function reported at screening.
- In the group receiving the vaccine plus KEYTRUDA, the risk of recurrence or death was reduced by 44% (hazard ratio, HR=0.56 [95% confidence interval, CI: 0.31 to 1.08]; p-value=0.0266) compared with the group on KEYTRUDA alone (acting as placebo). [Even though the study was open-label, the chance of the results being positive due to chance alone is less than 3%. A simple Google search of Moderna, Keytruda, and “confidence interval” turned up zero results from news outlets except for Evaluate Vantage, a specialist in pharma, biotech and medtech news. Thus, after 2 days of coverage after the initial press release, no one in mainstream media remembered enough basic statistics to recognize that because the CI crossed 1, the study results technically weren’t statistically significant.]
- mRNA-4157 is Moderna’s lead PCV (see Figure 1). It consists of a single synthetic mRNA coding for up to 34 neoantigens. [Neoantigens are unique proteins created by gene mutations in cancer cells that cause the development of tumors.] Upon administration, the mRNA uses the host body’s cell transcription/translation system to mass produce the neoantigens which hopefully provoke a quick and effective immune response, which is then directed to the developing tumors.
Figure 1. Moderna Pipeline
Moderna 2022 R&D Day presentation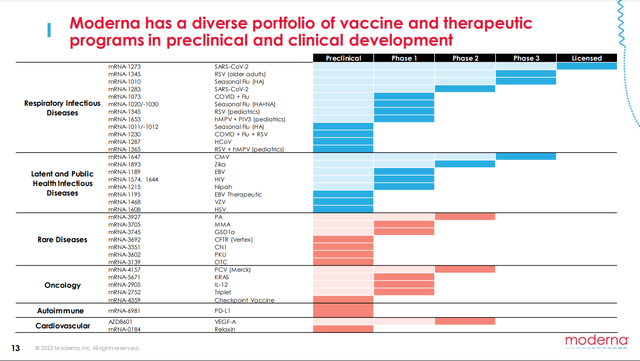
-
KEYTRUDA, Merck’s anti-PD-1 therapy, is indicated for many cancer types, [The pertinent one is that on February 15, 2019, the Food and Drug Administration (FDA) approved KEYTRUDA for the adjuvant treatment of patients with melanoma following complete resection. As an adjuvant, KEYTRUDA is given after initial treatment (the resection), to suppress secondary tumor formation. Approval was based on KEYNOTE‑054/EORTC1325, a double-blind, randomized, placebo-controlled, trial (“RCT”) in 1019 patients with completely resected, stage IIIA (>1 mm SNL metastasis), IIIB or IIIC melanoma. Inclusion criteria was similar to KEYNOTE-942. The PEP was RFS. Patients receiving KEYTRUDA experienced 43% fewer recurrences/deaths compared to the placebo arm (26% vs 43%, HR= 0.57; 95% CI: 0.46 to 0.70; p<0.001).]
-
Serious treatment-emergent adverse events (TEAEs) occurred in 14.4% of patients who received the combination arm of PCV plus KEYTRUDA versus 10% with KEYTRUDA alone. [People may question how a 44% improvement could be non-significant. Conversely, there was a 44% increase in TEAEs found. Given the choice, most would likely prefer a better chance to not get the cancer in exchange for a few more side effects.]
Takeaways
Because of the observed positive trend, it would be strategic for Moderna and Merck to go for a quick approval of mRNA-4157 with one well-designed Phase 3 trial in melanoma. There is of course a chance of failure in that future, but it’s unlikely. Skeptics could point to disappointing results presented on a competing PCV from Roche (OTCQX:RHHBY) and BioNTech (BNTX) at the 2020 American Association for Cancer Research Annual Meeting. In one of two first-in-human Phase 1 studies, RO7198457, also known as BNT122, only 1 patient out of 26 (overall response rate, ORR: 4%) had a response, but it was a complete response (“CR”). Meanwhile, in the other trial, the PCV in combination with Roche’s PD-L1 antibody, Tecentriq, only elicited responses in 9 of 108 patients (8% ORR, including 1 CR). However, Tecentriq is a weaker, more toxic antibody not indicated for monotherapy in unresectable or metastatic melanoma, and even its combination isn’t a preferred regimen per NCCN.
In comparison, the only other trial for mRNA-4157 is the KEYNOTE-603 on solid tumors. At the Society for Immunotherapy of Cancer 2020 meeting, an update reported 3 CRs and 8 partial responses out of 63 patients with various cancers who received a combination of mRNA-4157 and Keytruda. Of the 10 participants with head and neck squamous cell carcinomas (HNSCC), the ORR was 50% with 2 CRs, 3 PRs, and 4 others with stable disease (“SD”). Thus, the highlights were a disease control rate (CR + PR + SD) of 90%, as well as a median progression free survival (mPFS) of 9.8 months, which seems much better than Keytruda’s published ORR and mPFS of 14.6% and 2.0 months respectively, as monotherapy.
Financially, MRNA is a decent buy as a defensive healthcare stock in a bearish market. Moderna is also profitable with $17 billion in cash, $3.4 billion in revenue, and $1 billion net income for the third quarter. Despite the 20% gain on December 13, the company’s valuation was still A-rated on Seeking Alpha. However, the Quant system may not accurately capture the potential worth of the pipeline. KEYNOTE-942’s principal investigator said that these data provide the first randomized evidence that a personalized neoantigen approach may be beneficial in melanoma, and the first that improve on the rates of RFS achieved by PD-1 blockade in resected high-risk melanoma.
Investors should have confidence that management will plan and initiate a series of trials, not only the Phase 3 in melanoma, but in other tumor types such as HNSCC as well. It would save some money if Merck decides to partner, but Moderna, Inc. has the cash and doesn’t need to share the profits if it goes forward alone.


Be the first to comment