Prykhodov/iStock via Getty Images
Intrinsic Medicine, Inc. (INRX) is a preclinical stage synthetic biology therapeutics developer. The Company‘s product candidates are based on human milk oligosaccharides (‘HMO) that can modulate the gut microbiome as well as human cells and the immune system. Comprising over 200 oligosaccharides, the third most abundant solid component of human milk, HMOs have a structural diversity with distinct bioactivity that can be utilized – so says this preclinical company – to develop targeted new drugs for treating specific gut-brain axis (‘GBA) disorders.
The company‘s scientists have developed a process for manufacturing these molecules that are identical to those found in human breast milk. Synthesized human identical milk oligosaccharide (‘HiMO) molecules have been shown to be effective at treating a variety of diseases, including gastrointestinal disorders, respiratory infections, and cancer. The potential benefits of using HiMOs as treatments are significant. They can be administered orally as well as intravenously and, unlike traditional drugs, are not subject to side effects. What‘s more, because they are derived from human breast milk, they are unlikely to cause an immune response in patients. This could make them an ideal treatment option for patients who have failed other therapies or who cannot tolerate traditional medications.
The therapeutic potential of HiMOs is based on their ability to modulate the gut microbiome, which has been linked with a wide range of diseases. The first indication that the Company is targeting is inflammatory bowel disease (‘IBD), a group of chronic conditions that affect the digestive system. IBD patients have an altered gut microbiota that contributes to inflammation in the intestine. By administering HiMOs orally, the Company hopes to restore balance and reduce inflammation in these patients.
There is also evidence that HiMOs may be beneficial for other disorders associated with gut dysbiosis, such as obesity and type 2 diabetes. One study showed that obese mice treated with a HiMO-based supplement lost more weight and had lower blood sugar levels than control mice fed a standard diet. These results suggest that HiMO-based therapies could also be used as novel treatments for obesity and type 2 diabetes in humans. The Company‘s approach has several advantages over existing treatments for IBD, and perhaps obesity, and type 2 diabetes:
-
HiMO molecules are manufactured using synthetic biology, which allows for precise control over their composition;
-
They are derived from human milk oligosaccharides (HMOs), which are natural ingredients shown to be safe and effective in clinical studies;
-
Unlike other medications used to treat these diseases, HiMO molecules do not alter the gut microbiota indiscriminately; instead, they selectively target pathogenic bacteria while leaving healthy bacteria intact.
HiMOs have already been shown to be effective against a number of diseases. In particular, they have been shown to be effective against both rotavirus and norovirus, two major causes of diarrhea in children. Additionally, HiMOs may also prove useful in the treatment of other conditions such as cancer and autoimmune disorders.
Pipeline & Trials
The Company believes “HiMO drugs can have a favorable toxicity and tolerability profile in the therapeutic context,” because “HMOs have been selected and conserved over millions of years of mammalian evolution, with all human beings exposed before birth and during early life development.” The Company has only recently produced individual HMO-equivalent Oligosaccharide Medicines (‘OMs) at a scale sufficient for therapeutic development and potential commercialization. The Company has “selected these drug candidates based on published third-party clinical, preclinical and toxicology data indicating the potential for disease-modifying HMO bioactivity combined with a low risk of dose-limiting toxicity.“
Pipeline (Company website)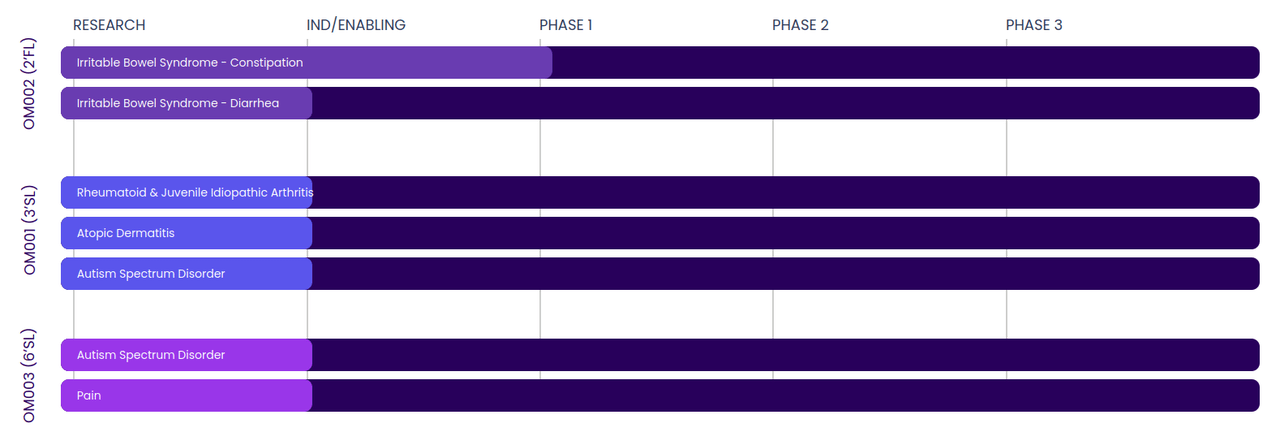
The Company‘s lead drug candidate is OM002 (2‘FL or fucosyllactose) which will be evaluated for the treatment of constipation predominant irritable bowel syndrome (‘IBS-C), in the Company‘s first phase 2 clinical trial to be initiated in 1H-2023 in Australia. Additionally, based on the feedback received from the US FDA during a pre-IND interaction, the Company will conduct its own confirmatory toxicology studies for data to be included in its IND application anticipated in 1H-2023 to initiate phase 2 trial in the US. OM002 will also be evaluated for treatment of the diarrhea predominant IBS (‘IBS-D). If approved, OM002 could potentially be the first drug to address both IBS-C and IBS-D, estimated to affect over 10 million people in the US, and around 1 in 10 people globally.
OM001 (3‘SL or sialyllactose) is being advanced in Rheumatoid Arthritis & Juvenile Idiopathic Arthritis for which IND/CTA is to be submitted for initiation of phase 1b trial. Pre-IND interaction with FDA is planned for supporting pilot trials in Atopic Dermatitis and Autism Spectrum Disorder (‘ASD).
OM003 (6‘SL) is being advanced for ASD and pain. Preclinical research is to be completed before pre-IND interaction with FDA supporting pilot trials.
Management
The Company‘s management team includes – co-founder, Chairman and CEO Alexander Martinez, who has over 16 years of healthcare experience with most of it at Ionis Pharmaceuticals, Inc., and Akcea Therapeutics, Inc.; co-founder, President and COO Jason Ferrone, who has been in drug discovery and development for over 20 years, half of it being at Ionis as clinical lead as well as head of regulatory affairs; CMO Emil Chuang, M.B., B.S. (SYD) FRACP, is a pediatric gastroenterologist, with academics from University of Sydney, and initial career at Duke University, and University of Pennsylvania. Dr. Chuang has over 20 years of industry experience including Centocor Biotech, Inc. (now known as Janssen Biotech, Inc.), Nestle Health Science S.A., Takeda Pharmaceutical Company, Ltd. and Progenity, Inc.
Financials
The Company was originally incorporated as Lupa Bio, Inc. in August 2018 and subsequently changed its name to Intrinsic Medicine, Inc. in August 2020. A wholly-owned subsidiary, Intrinsic Medicine AU Pty LTD, was formed in October 2021. The Company has accumulated a deficit of approximately $14.3 million as of 12/31/2021, and its cash balance of $2.77 million is not sufficient for operating the company for the next 12 months. The Company has filed a Form S-1 registration statement for an initial public offering (‘IPO) of an unspecified number of shares of its common stock not priced yet, and applied to list its common stock on the Nasdaq Capital Market under the symbol INRX. The registration also includes shares to be sold by certain selling stockholders.
Risks
Intrinsic Medicine is an “emerging growth company” and a “non-accelerated filer” and a “smaller reporting company” pursuant to which the Company may take advantage of exemptions from reporting requirements.
The Company has a limited operating history, and is at a very early stage of development. It will be several years before they complete clinical trials and reach the regulatory stage.
The Company has suffered losses since inception, accumulating a deficit of approximately $14.3 million as of 12/31/2021. The Company does not have a cash runway for 12 months and unless funding is met with the IPO or any other means, the Company may not be able to continue as a going concern.
There is substantial competition not only from larger and better financed private and public healthcare companies, but also from non-prescription products marketed as dietary supplements.
The Company‘s OM drug candidates are intended to be a prescription-only form of a naturally occurring HMO, hence IP protection may be limited to method of use, leaving the market wide open for competitors. Furthermore, the Company is highly dependent on Glycosyn as a licensor of its main drug candidates, the rights to which Glycosyn itself has acquired via an exclusive, sublicensable, worldwide in-license from the patent owners, Cincinnati Children‘s Hospital Medical Center and the other licensors thereunder, or the Glycosyn In-License, and for enabling manufacturing technology related to the synthesis of OM drug candidates. The Company is also highly dependent on The Regents of the University of California, through its San Diego campus, The University of California San Diego, or UC San Diego, as a licensor of certain of its main drug candidates.
The Company is wholly dependent on third-party suppliers for clinical and commercial supplies, including the active ingredients which are used in its drug candidates. It also relies on a sole supplier, Royal FrieslandCampina N.V., a Dutch multinational dairy cooperative based in Amersfoort, Netherlands, and its subsidiaries, for certain of its supplies, and intends to continue relying on the same.
Bottomline
Leveraging synthetic biology-manufactured HiMO molecules as new medicines could revolutionize the way we treat a variety of diseases. If further research and trials confirm their efficacy against other diseases, this could lead to a whole new class of drugs that are safer and more effective than current treatments available. Intrinsic Medicine is currently at an early stage with its HiMO molecule therapy and the next few years are very crucial for them. While targeting the human gut microbiome is interesting, we don’t have any investment interest in this early stage company at this time.


Be the first to comment