Orbon Alija/E+ via Getty Images
On January 24, Cassava Sciences (NASDAQ:SAVA), a large ($1.23 billion market cap) biotechnology company, announced positive top-line results for simufilam, its oral drug candidate for Alzheimer’s disease (“AD”) dementia, in PTI-125-04, a Phase 2 open-label safety (“OLS”) study with exploratory 12-month efficacy endpoints. Over 200 patients with mild-to-moderate AD (16-26 score on Mini-Mental State Examination) were enrolled and received simufilam 100mg tablets twice daily for 1 year or more. Shares tanked -19% on the day and are still trending downward as the market likely perceives the news as a ‘reversion to mediocrity’ of the scores on the 11-item AD Assessment Scale-Cognitive (ADAS-Cog11) compared to previous interim analyses (Table 1; higher scores mean worse performance). Investors should be fully informed on the real implications of the data.
Table 1. Improvements (lower is better, negative numbers in parentheses) on ADAS-Cog in simufilam open-label safety study
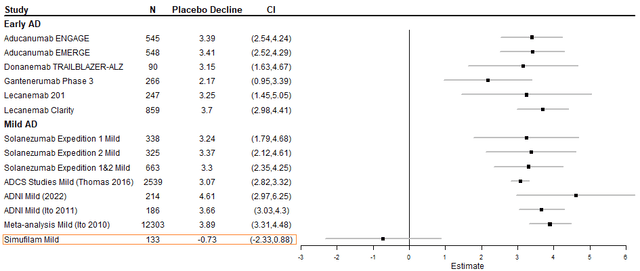
The Cassava highlighted that after a year on simufilam, 47% of patients improved on ADAS-Cog by a mean of -4.7 points, and an additional 23% of patients declined by fewer than 5 points. The company likes to contrast that to the average decline of 5.5 points over 12 months in mild to moderate AD patients determined by a 2010 meta-analysis. It turns out that calculating mean decline for the 53% of patients for whom the drug didn’t work by subtracting from the total population score (0.5 x 216) gives 5.11 points, and voila, it’s like the ‘non-responders’ took placebo. The modelings in Figures 1 and 2 illustrate that management, if it can, should probably stick to mild disease for quicker approval.
Figure 1. Model of historical placebo declines on ADAS-Cog in patients with mild-to-moderate AD.
Figure 2. Model of historical placebo declines on ADAS-Cog in early disease and mild disease.
To add more color, the best performing placebo subgroups were examined from trials of approved AD drugs, including donanemab, which would’ve been had Eli Lilly and Company (LLY) paid more attention to its trial. If the ADAS-Cog scores were specified, it was added to Table 3. There is evidence that the apolipoprotein E (ApoE) ε4 allele increases the risk of AD by promoting earlier and more abundant amyloid deposits in the brain, but it’s unclear if it has any relevance to simufilam. Overall, it seems simufilam has little to fear from a placebo effect, even one of a magnitude that sank Roche’s (OTCQX:RHHBY) gantenerumab.
Table 3. Subgroups with the least declines on ADAS-Cog compared to Active Drug
|
Subgroup |
N (Placebo, Treatment) |
Decline |
Treatment |
|
|
Laboratory ApoE ε4 Noncarrier |
178, 182 |
2.6 |
2.8 |
|
|
ENGAGE |
Laboratory ApoE ε4 Carrier |
378, 379 |
3.1 |
3.5 |
|
Mild AD |
46, 32 |
5.36 |
3.172 |
Turning to the drugs that were actually approved, Aduhelm from Biogen (BIIB), Leqembi from Eisai (OTCPK:ESALY), and the still-in-the-running donanemab, simufilam would probably command the lion’s share of the market, given its relative safety alone, specifically, the lack of toxicities inherent in the amyloid-lowering therapies. Of course their respective trials can’t be directly compared, but in the OLS, ‘mild’ patients (MMSE 21-26) improved by -2.4 points, so there is a bit of leeway compared to the ‘mild’ Model, which still stands above the field (Table 4). Even in totality, how good is simufilam’s 0.5 point ‘decline’? For the ADAS-Cog, a 2-point decline over 12 months was suggested as a threshold for “minimal-worsening” and 3-4 points for “moderate-worsening”. Therefore, clinicians wouldn’t be able to distinguish such a miniscule slide.
Table 4. Model of historical Active Drug declines (improvement) on ADAS-Cog in early disease and mild disease.
|
Study |
Approved dose (investigational) |
Study duration |
N |
Active Drug Decline |
|
PTI-125-04 |
(simufilam) |
12 months |
133 |
(0.73) |
|
EMERGE |
Aduhelm 10 mg/kg every four weeks |
78 weeks |
547 |
2.60 |
|
ENGAGE |
Aduhelm 10 mg/kg every four weeks |
78 weeks |
555 |
3.10 |
|
(donanemab) |
52 weeks |
93 |
1.53 |
|
|
(donanemab) |
76 weeks |
93 |
2.91 |
|
|
lecanemab 201 |
Leqembi 10 mg/kg biweekly |
79 weeks |
152 |
2.59 |
|
non-missing data only |
Leqembi 10 mg/kg biweekly |
79 weeks |
79 |
1.61 |
|
MCI due to AD |
Leqembi 10 mg/kg biweekly |
79 weeks |
47 |
1.93 |
|
Mild AD |
Leqembi 10 mg/kg biweekly |
79 weeks |
32 |
3.05 |
|
Leqembi 10 mg/kg biweekly |
18 months |
854 |
4.14 |
To paraphrase Suzanne Hendrix, PhD, and CEO of Pentara (a biostatistical consulting company specializing in neurodegeneration including AD), who is staking the reputation of herself and her company, “The improvement in ADAS-Cog over 1 year in mild patients taking simufilam is well outside the expected range of historic approved (or should’ve been approved) drug decline rates from numerous other studies.” In the meantime, much of the thesis of the previous article remains. Cassava has a lot of cash, no debt, low enough burn so far and thus can fund operations for at least a year, especially since the OLS didn’t fail. Now all eyes will be on the readout of the Phase 3 RETHINK-ALZ later in 2023.
To conclude, there are at least two viewpoints that could lead investors astray: 1) expecting a drug to reverse AD without a mechanism to reverse the aging process; 2) thinking simufilam performed like placebo in the OLS (possibly driven by the lack of a p-value). If the market continues to overreact, traders can profit on volatility by buying whenever the stock is oversold and sell on the rebound. Options will also be attractive once timelines become clearer. To be sure, simufilam still has heavy lifting to do. If Cassava is pursuing a label including early AD, improving or slowing cognitive impairment is one-half of the FDA’s requirement, along with allowing the patient to maintain daily functions. There is no published guidance from the Agency regarding AD with overt dementia, but the Phase 3 trials do have Alzheimer’s Disease Cooperative Study – Activities of Daily Living (ADCS-ADL) scale as a co-primary endpoint. There has been no data here, but a vast majority of items on the ADCS-ADL such as locating belongings, figuring out steps in processes, and remembering routines are affected by cognition, so investors can have a degree of confidence that simufilam will do fine in this area.



Be the first to comment