ferrantraite/E+ via Getty Images
On Monday, Oct 5, BioVie (NASDAQ:BIVI) announced results from two phase 2 trials studying their lead candidate NE3107 for the treatment of Parkinson’s Disease (PD) and Alzheimer’s disease (AD).
On Oct 6, BIVI announced additional data from their p2 AD trial.
Two days later, on Oct 7, the company issued a letter to the shareholders to summarize the results.
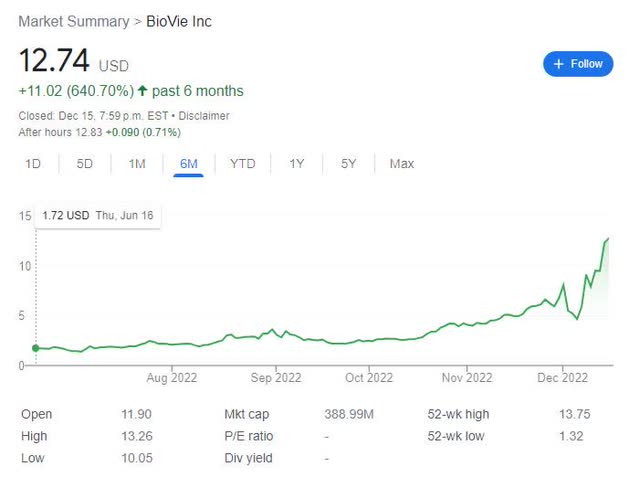
The stock has rallied after the news. As seen below, in the past month, the stock has risen >150%; or in the last 6 months, >600% from $1.72 of June 16, 2022.
Google-BIVI 1 month chart Google-BIVI 6 months chart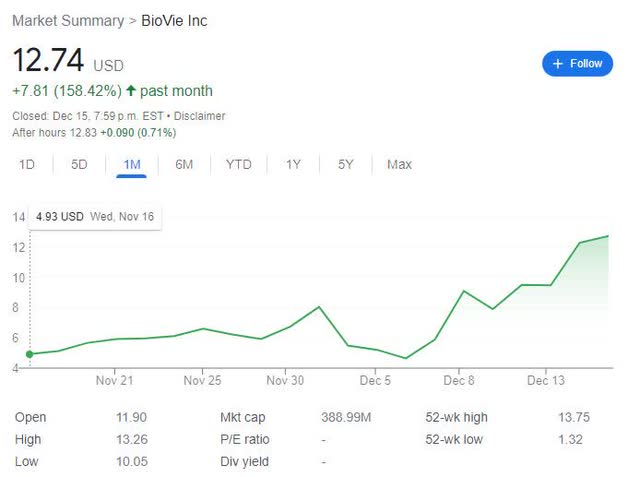
Let’s now turn to the results.
BIVI’s P2 Parkinson’s Disease Trial Data
First, the basics: it’s a double-blind, placebo-controlled, safety, tolerability, and pharmacokinetics study in Parkinson’s disease patients, treated with levodopa and NE3107 combined vs. levodopa alone [placebo arm], for 28 days (n=45).
The trial protocol has 13 prespecified primary endpoints.
For readability, I list only the headings and not the details of each of these 13 primary endpoints (bold added for emphasis)
| 1. Change from baseline in Motor disease society- Unified Parkinson’s disease rating scale (UPDRS) Part III total score for the patient in the “off-state” (without L-dopa for previous eight hours) |
| 2. Change from baseline in the length of time during which L-dopa therapy is ineffective in reducing motor symptoms of disease (OFF time) |
| 3. Change from baseline in average Motor disease society- Unified Parkinson’s disease rating scale Part III total score measured over the course of 8 hours after taking L-dopa and/or NE3107 |
| 4. Motor disease society- Unified Parkinson’s disease rating scale Part I total score |
| 5. Motor disease society- Unified Parkinson’s disease rating scale Part 2 total score |
| 6. Change from baseline in the length of time during which L-dopa-like effects are felt by the patient |
| 7. Change from baseline in L-dopa induced dyskinesia measured with the abnormal involuntary movement scale |
| 8. Change from baseline in time to onset of L-dopa-like activity |
| 9. Change from baseline in Non-Motor Symptom Assessment Scale for Parkinson’s Disease |
| 10. Change in area under the levodopa plasma concentration vs. time curve when administered alone compared to being co-administered with NE3107 |
| 11. Change in the maximum plasma concentration of levodopa when administered alone compared to being co-administered with NE3107 |
| 12 The area under the NE3107 plasma concentration vs. time curve when administered alone compared to being co-administered |
| 13. Change in the maximum plasma concentration of NE3107 when administered alone compared to being co-administered with L-dopa |
The table below lists Oct 5 PR’s headline and the sub-headlines.
| NE3107-treated patients experienced greater motor control in Parkinson’s trial |
|
(Source; bold added for emphasis)
Regarding the two objectives, further in the PR, the company explains:
This trial was launched with two design objectives: 1) the primary objectives are safety and a drug-drug interaction study as requested by the FDA to demonstrate the absence of adverse interactions of NE3107 with levodopa; and 2) the secondary objective is to determine if preclinical indications of promotoric activity and apparent enhancement of levodopa activity can be seen in humans.
Two comments:
1. It’s unknown currently whether or not the other 11 pre-specified endpoint results support or contradict BIVI’s claims that the trial has met its two “design objectives”, as those results are not disclosed.
2. The so-called “clinical-meaningful” “superiority” [UPDRS part 3] data, comparing two arms or un-prespecified subgroups by age, are not statistically-significant, which means that they are not a reliable source to draw any conclusion.
For example, the headline that states “NE3107-treated patients experienced greater motor control in Parkinson’s trial” is poorly or un- supported, by the reported p2 data so far, in my opinion.
BIVI’s P2 Alzheimer’s Disease Trial Data
Previously (on Sept 7 2022), BIVI has reported results from their phase 2 AD trial. Two recent updates were announced on Oct 5, and on Oct 6.
This phase 2 AD trial is an open-label, single-arm study that enrolled a total of 23 patients, which includes eighteen patients with Mini-Mental State Examination (MMSE) scores ≥ 20 (mild cognitive impairment to mild AD), and five patients with MMSE <20 (moderate AD).
The enrolled patients were treated with 20 mg of NE3107 twice daily for 3 months.
The table below lists the AD trial’s pre-specified endpoints. As previously, only the headings (except the first* endpoints), are listed.
| Primary Outcome Measures |
|
*1. fMRI MRS Change in MRS from baseline to completion on advanced functional magnetic resonance imaging (fMRI). Specifically, the change in glutathione levels will be analyzed (as measured by Magnetic Resonance Spectroscopy (MRS) |
| 2. fMRI DTI-NODDI Change |
| 3. fMRI ASL Change |
| 4. fMRI resting BOLD Seed Change |
| 5. fMRI NVR Change |
| Secondary Outcome Measures |
|
*1. Clinical Dementia Rating [CDR] Change as calculated from the Quick Dementia Rating Scale Change Quick Dementia Rating Scale (QDRS) The Quick Dementia Rating Scale (QDRS) is an interview-based tool administered by study officials to participants’ caregivers used to obtain observations from a consistent source. The QDRS form consists of 10 categorical questions (5 cognitive, 5 functional), each with 5 detailed options depicting the level of impairment as either 0 (normal), 0.5 (mild/inconsistent impairment), 1 (mild/consistent impairment), 2 (moderate impairment), or 3 (severe impairment). Based on the conversion table outlined in Dr. James Galvin’s research (2015), total QDRS scores were converted to Clinical Dementia Rating (CDR) scale levels ranging from 0 (normal aging), 0.5 (mild cognitive impairment), 1 (mild dementia), 2 (moderate dementia), and 3 (severe dementia). |
| 2. Montreal Cognitive Assessment (MoCA) Change |
| 3. Alzheimer’s Disease Assessment Scale – Cognitive Subscale (ADAS-Cog) Change |
| 4. Mini-Mental State Examination (MMSE) Change |
| 5. Glucose Serology/Metabolic Level Change |
(Source: Compiled by author, emphasis added)
Two notables:
1. All five of primary endpoints and the last of secondary endpoints are biomarker data.
2. Cognition measurements are secondary endpoints, which include the CDR change being the first endpoint. However, per protocol, the CDR change is not a direct measurement, but a calculated/converted result from another measurement, namely QDRS.
Having seen the pre-specified endpoints, let’s now turn to the announcements.
| From Oct 5 PR |
|
| Additional data from Oct 6 PR |
| The resulting data for patients treated with NE3107 for three months showed a reduction of 3.3 years (p=0.0021) on the Horvath DNA methylation SkinBlood clock. Furthermore, 19 out of the 22 patients experienced this reduction in the SkinBlood clock score. |
(Compiled by author; emphasis added)
Few notables:
1. For an open-label [uncontrolled] study, which had five primary biomarker endpoints, the announcements have switched the trial’s pre-specified priority, namely from primary (biomarkers) to secondary (cognition measurements), and reported/emphasized accordingly.
2. Given the fact that it’s an single arm, open-label trial (no placebo control) in this trial, the statistically-significant “improvement” cognition data at 3 months, from the patients’ own baseline, cannot be attributed entirely to the treatment effect. Namely, there is no reliable way to separate the treatment effect from the placebo effect.
3. Of four cognition measurements reported, i.e., ADAS-Cog, CDR, ADCOMS, Global Rating of Change, only the first two are per protocol and as noted before, CDR results are not a direct measurement, but calculated/converted results from QDRS, according to the protocol.
BIVI did not disclose Montreal Cognitive Assessment (MoCA) and MMSE results, and therefore it’s unknown whether these measurements support or contradict the other cognition data.
4. The four (biomarker & other) data, mentioned or reported, i.e., TNFα, CSF phospho-tau level, the ratio of p-tau to Aβ42, and the “Horvath DNA methylation SkinBlood clock”* score change are not pre-specified, per protocol endpoints.
Among the five pre-specified biomarker endpoints, only the first, i.e., advanced functional MRI results, was mentioned.
*Note: the “Horvath DNA methylation SkinBlood clock” measurement is not a per protocol endpoint in BIVI’s current p2 AD trial, on-going p3 AD trial, or any other AD trials that I’m aware of. Its validity and usefulness as supportive data in the NE3107 data package are questionable, in my opinion.
5. What happened to the 23rd patient?
Discussion
On Oct 7, BIVI issued a letter to the shareholders to summarize these results:
Firstly, as commented earlier, “clinically meaningful increase in motor control” [from the PD trial] is poorly or un-supported, as there are no statistically-significant results reported from BIVI’s placebo-controlled p2 PD trial.
For example, Annovis’ (ANVS) placebo-controlled p2 PD trial (n=54; duration=25 days) showed a statistically-significant UPRS, Part 3 improvement over patients’ own baseline (though not over placebo). See below.
ANVS Dec 2022 Presentation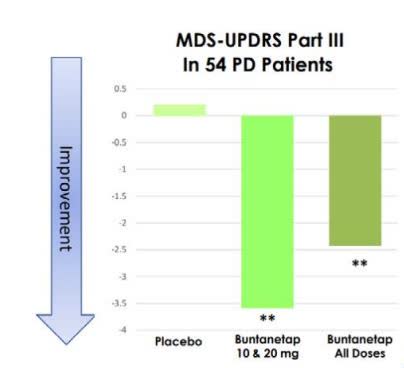
(Source; from slide 14)
Secondly, BIVI’s open-label p2 AD trial, per protocol, is primarily about biomarker data, though the company did not report on the four out of five pre-specified biomarker data.
It should be noted that for AD trials, biomarker data, though important/supportive, are un-reliable substitutes for cognition efficacy data, e.g., accelerated approval of Aducanumab. Nor is the correlation between biomarkers and cognitive data a pre-specified endpoint.
This is because pre-specified biomarker data, however positive, cannot be used to evaluate if or to what extent any observed cognitive improvement is due to the treatment or the placebo effect. Only by comparing cognitive data between the arms (treatment vs. placebo) directly, can the real cognition efficacy data be established.
Thirdly, the so-called “enhanced cognition” data from BIVI’s p2 AD trial, a small, open-label, uncontrolled trial of a short duration (3 months), needs to be considered in context. Namely, they are not efficacy data from a well-powered, well-controlled trial of a long duration. At best, this data could point to early, “probable” signal, that needs to be established in a larger, longer, controlled trial.
For example, the need for a placebo arm can be seen in other AD trials, such as ANVS’ small, placebo-controlled AD trial. ANVS’ drug showed a statistically-significant improvement in ADAS-Cog change at 25 days, over patients’ own baseline, but not over the placebo.
ANVS Dec 2022 Presentation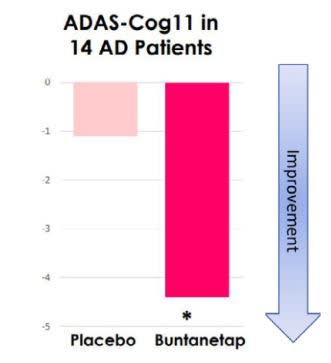
(Source; slide 15)
In summary: BIVI’s PD trial (n=45, 28 days) generated no statistically-significant results; and the AD trial (n=23, 3 months) has no placebo control to meaningfully measure the treatment effects.
This means, no data from either trial, should be given undue weight, in terms of how positive they really are. They are far from being conclusive.
Financials
According to the latest 10-K filing (Page 30-31), the net loss ended in June 2022 is $26.1M and as of June 30, 2022 “the Company had working capital of approximately $14.6 million, cash of approximately $18.6 million.”
Conclusion
As I comment recently in my article on AVXL, also an AD/PD drug developer, I appreciate deeply the dedication of companies & investors in this space, as they endeavor to develop safe & effective treatments to meet such serious, unmet medical needs.
Regarding BIVI’s p2 PD & AD trial results, I think perhaps the investors’ enthusiasm has gotten ahead of the results.
Yes, these are probably positive results, but a >600% in 6 months or >150% in one month increase in valuation worth of positivity, I’m not sure.
For example, two peers in the same space, ANVS, and INMB, that are much more modestly priced, also reported “positive” early data from their respective trials.
| Positive results |
Market Cap on 2022-12-15 |
|
| BIVI | p2 PD/AD trials | $414 M |
| ANVS | p2 PD/AD trials | $99 M |
| INMB | p1b AD | $135 M |
(Compiled by author, from SA info)
It is possible that this enthusiasm was partly due to the speculative hype for the upcoming p3 AD trial topline (n=400, duration=30 weeks) that BIVI expects by mid-2023.
If that is the case, one must remember that “positive” early (p1, p2) data, regardless of how “positive” they may seem, is still a long way from a successful p3 readout.
Thanks for reading. All the best!



Be the first to comment