Aquestive Therapeutics (AQST) has been a roller coaster investment over the past year, but 2020 is setting up to be a transformative year for the company and investors. The company laid the groundwork in 2019, with a pre-IND meeting for AQST-108, the FDA accepted Libervant’s NDA, and the company is reporting continued growth for SYMPAZAN. Now, investors can look forward to apomorphine’s PDUFA date on May 21st and Libervant’s PDUFA on September 27th, both of which should unlock substantial revenue for the company. I believe the market will start to recognize Aquestive’s potential, and as a result, I still see AQST as a great speculative buy at these current prices.
I intend to review Aquestive’s recent success and discuss why 2020 will be a transformative year for the company. In addition, I reveal my plans for my AQST position.
AQST-108 Moving To The Clinic
Aquestive met with the FDA back in February regarding the company’s buccal epinephrine candidate, AQST-108. The FDA determined that AQST-108 will be reviewed under the 505B2 pathway and no additional studies will be needed for the IND application. As a result, AQST will have a straightforward path to approval, which could be faster and fairly inexpensive. The company expects to open the IND in Q2 and initiate its PK clinical trials before the end of 2020.
If all goes well, AQST could have one of the first non-subcutaneous or intramuscular injection treatment for anaphylaxis.
Libervant Has A PDUFA
Aquestive filed Libervant’s IND in late November and now has a PDUFA of September 27th. If approved, Libervant could be the original orally administered product for breakthrough or cluster seizures. The company believes that Libervant could generate up to $300 million in peak net revenues within four years after launch.
On January 13th, the FDA approved Neurlis’ Valtoco, a diazepam nasal spray for acute treatment of intermittent, stereotypic episodes of frequent seizures. As a result, Libervant would not be the first product on the market for a very similar indication. What is more, Valtoco has an orphan drug designation, which could prevent Libervant from hitting the market for almost seven years. So, investors need to accept that Libervant’s launch may not be in the near future.
SYMPAZAN Laying The Groundwork
Aquestive developed and launched SYMPAZAN as a commercial forerunner, for Libervant and laying the groundwork for neurological PharmFilm products. SYMPAZAN prescribers should also be potential Libervant prescribers, so hopefully, they are comfortable with Aquestive’s PharmFilm.
According to the company, shipment volume has been up since the beginning of the year and the number of prescribers has grown over 44% since Q3, with about 78% of those prescribers writing multiple scripts. Aquestive believes it has an 18% penetration rate and has over 70% of lives covered at the end of 2019. If the company continues to execute, it expects SYMPAZAN will eventually hit $65 million in peak annual net sales.
Indeed, $65 million in peak sales is not something to get really excited about, but investors should understand that SYMPAZAN is supposed to set up Libervant for commercial success and not be a cash cow.
Making The Transition
Aquestive’s traditional source of revenue has been its Suboxone business. However, that stream is starting to slow, which has the company creating new sources of revenue. My long thesis has been based on Aquestive transitioning into a commercial-stage company by pushing its own products. Although the road hasn’t been perfectly smooth, I believe the company has managed the transition very well.
I believe Aquestive’s management of the financials will determine if and when an AQST investment will be fruitful. Recently, the company raised over $40 million and started 2020 with ~$49 million in cash, which can be used to help commercialization efforts and progressing pipeline programs. Aquestive continues to manage its costs and believes it has the capital required through early 2021. What is more, the company anticipates a $4 million milestone from Sunovion and will look to monetize its rights to the apomorphine product once approved, which should extend the cash runway. Once the company has the finances, it can focus on advancing AQST-108 and commercial activities.
Yes, it has already crossed the line and is now a commercial company, but I need to see Aquestive hit a few more milestones before I consider the transition to be a success.
First and foremost, the approval and launch of Libervant… considering the uncertainties around these events, I have to say that Aquestive’s transition to a proprietary commercial-stage company is partial.
Downside Scenario
The downside risks mentioned above does create a potential domino scenario that could punish the share price. Firstly, the potential that Libervant isn’t approved, or isn’t allowed, to hit the market due to Valtico’s orphan drug designation. If the company is unable to get Libervant on the market, we should expect a strong sell-off. Unfortunately, I don’t expect a strong bounce following the potential sell-off due to the shrinking revenue stream from Suboxone, which will obviously prolong the company’s cash burn.
Still A Buy?
Despite the potential downside risks, I still see AQST as a speculative buy for a number of reasons. Primarily, the company’s current valuation for its current and forward revenue/sales. The Street expects Aquestive to record approximately $41 million in 2020, which is a forward price-to-sales of 2.96 (Figure 1). Considering the sector’s average price-to-sales is roughly 5x, we can say AQST is currently discounted.
Figure 1: AQST Revenue Estimates (Source: Seeking Alpha)
The forward price-to-sales is expected to only improve in the subsequent years as the company continues to move deeper into commercialization and additional products hit the market.
Another positive aspect is AQST’s technicals, which shows the share price breaking out of a long-term downtrend (Figure 2).
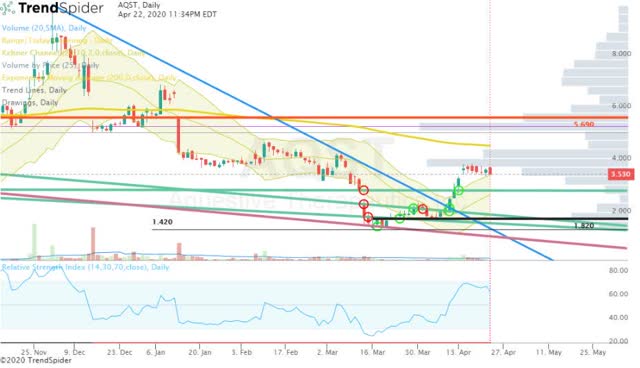
Figure 2: AQST Daily (Source: TrendSpider)
If the share price is able to break above the 200-day EMA (yellow line), we could see a quick return to the $5.00 area.
Last but not least, I believe Aquestive’s platform technology will continue to produce impressive pipeline candidates that will dethrone current standard-of-care products. Once the company has a few commercial products on the market, I believe the market will be forced to acknowledge its potential, and the share price will start to reflect the opportunity.
What’s My Plan?
Despite the Coronavirus Crash and Libervant uncertainties, I’m going to stick to my previous plan of making multiple additions to my AQST position throughout 2020 and expect to leave the year with a full position. I was going to time my additions around key catalysts, but I am going to return to technical analysis to determine my buys. I will wait and see if the stock wants to test the $2.00 area again before or after apomorphine’s PDUFA. If the FDA sends a CRL, I will wait for the sell-off to subside and add. I will make the remaining additions once Libervant’s fate is decided by the FDA. Once I have had my fill, I expect to hold AQST for at least five years in anticipation of a large return on investment or possible acquisition.
Disclosure: I am/we are long AQST. I wrote this article myself, and it expresses my own opinions. I am not receiving compensation for it (other than from Seeking Alpha). I have no business relationship with any company whose stock is mentioned in this article.


Be the first to comment