davelogan/iStock via Getty Images
There are currently no cures for Alzheimer’s disease, but there may be some effective treatments. These potentially include Anavex’s blarcamesine, Cyclo Therapeutics Trappsol Cyclo, and panax ginseng. The evidence for each is at this point based on a small sample size, but to a certain degree bolstered by the underlying science. Some investors may consider case studies and small open label trials as too uncertain to literally or figuratively buy in, but the case will be made for each of the above listed treatments in this article. Comparisons will also be made between treatments that transiently improve cognition (up to one year) and treatments that modify Alzheimer’s disease (i.e. largely stabilize Alzheimer’s disease for more than one year) and how one can account for these differences.
The following chart visually establishes the contrast between transient improvements in cognitive function and more long lasting changes (AchE= acetylcholinesterase inhibitors).
Disease modifying (Taiwanese Journal of Psychiatry)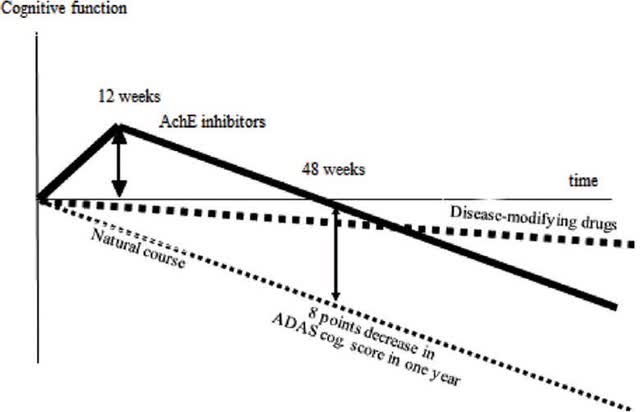
The next graph illustrates the difference between conventional therapies (acetylcholinesterase inhibitors and/or Namenda) and conventional therapies plus herbs. For comparison purposes, a one point change in Mini Mental State Examination scores (MMSE) is the equivalent of an approximately 2.3 point change in Alzheimer’s Disease Assessment Scale-Cognition (ADAS-Cog) scores.
Conventional therapy plus herbs (BMC Complement Alt Med)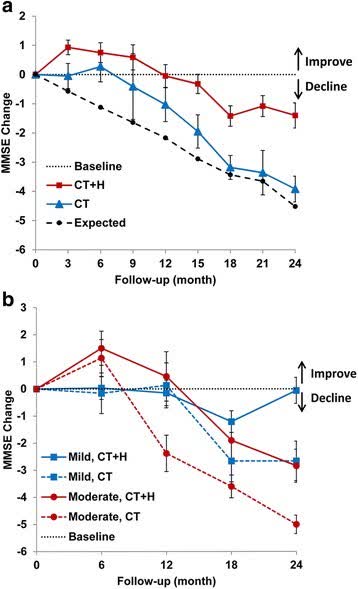
At one year in mild Alzheimer’s disease, the addition of antioxidant herbs makes no difference over conventional therapies. But after that in mild Alzheimer’s disease (and before that in moderate Alzheimer’s disease) the use of these herbs produces a significant benefit over conventional therapies. The following may explain why.
Acetylcholinesterase inhibitors are sigma-1 receptor agonists (due to their methyl and amine group) which by limiting the release of intracellular calcium inhibits acetylcholinesterase activity, the formation of amyloid, and the production of oxidants. The acetylcholinesterase inhibitor Aricept/donepezil contains a ketone group which acts as a peroxynitrite decomposition catalyst. Peroxynitrite is a nitro-oxidant that causes widespread damage in the brain during Alzheimer’s disease. Potentially, at least, amines in rivastigmine and galantamine can also be converted into ketones. For about a year in mild Alzheimer’s disease when acetylcholinesterase activity is high and peroxynitrite levels are relatively low inhibiting intracellular calcium release and accelerating the decomposition of peroxynitrite is enough to stabilize the disease. But as the disease progresses, more effective means of inhibiting the formation of peroxynitrite and of attacking it are needed.
Cassava Sciences’ (SAVA) simufilam produced similar results as acetylcholinesterase inhibitors at one year in a group of 50 participants with presumably mild Alzheimer’s disease (a .2 point decline in ADAS-Cog). Another 50 participants most of whom likely had mild cognitive impairment or very early Alzheimer’s disease did better (a 3.2 point improvement in ADAS-Cog). Simufilam may be a sigma-1 receptor agonist if its amide group is converted into an amine. Directly or indirectly amides can be converted into ketones, so simufilam may be a peroxynitrite decomposition catalyst as well. Its mechanism of action may then be the same as acetylcholinesterase inhibitors. Simufilam, though, may also be a copper and zinc chelator (via carboxylic acid) which would both unfold proteins (such as amyloid, tau, and filamin A) and reduce their role in overacting various receptors (zinc, copper). This would lower oxidative stress in Alzheimer’s disease, at least during its early stages. However, it is difficult to find a means by which simufilam modifies Alzheimer’s disease by scavenging peroxynitrite.
Peroxynitrite scavengers partially reverse the oxidative and nitrostative damage done to critical receptors, transport systems, and enzymes in the brain. They partially restart the regeneration of neurons, increase acetylcholine levels, and limit the death of neurons all of which are needed for the retrieval of memories. Inhibiting the formation of peroxynitrite, scavenging peroxynitrites, and reversing part of the damage that they do to the brain helps to ameliorate the disease.
The following may be disease modifying treatments for Alzheimer’s disease: i.e. treatments that largely stabilize the disease over long periods of time. The compounds responsible for the desired effects are noted in parenthesis:
Anavex’s (NASDAQ:AVXL) blarcamesine (Anavex 2-73)
Blarcamesine (Anavex Life Sciences)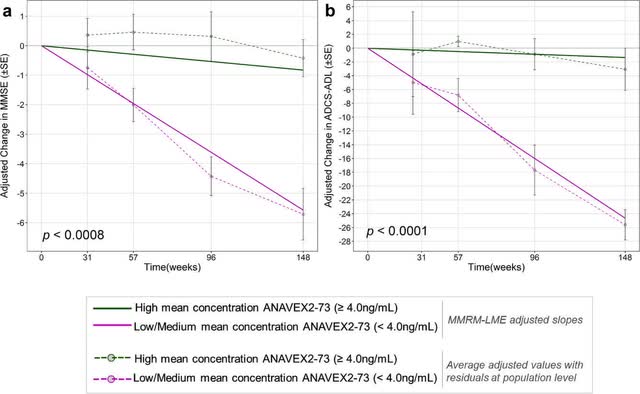
Anavex’s blarcamesine inhibits peroxynitrite formation via sigma-1 receptor agonism (amine and methyl groups) and probably scavenges peroxynitrite (tetrahydrofurans). The evidence for the latter is indirect: tetrahydrofurans donate electrons and hydrogen atoms which is essential for peroxynitrite scavenging and they scavenge hydrogen peroxide (many hydrogen peroxide scavengers are also peroxynitrite scavengers). A recent study showed that blarcamesine upregulated genes in Parkinson’s Disease and Alzheimer’s disease which improved pathways that helped to counteract neurodegeneration. Shortly after that a peer reviewed publication identified the multiple benefits of sigma-1 receptor agonism:
SIGMAR1 has emerged as one of the prominent targets in treating neurodegeneration. It is involved in modulating glutatmate levels, maintaining endoplasmic reticulum (ER) function, and regulating calcium. SIGMAR1 activation promotes neurogenesis, reduces reactive oxygen species (‘ROS’) formation, suppresses neuroinflammation, and ameliorates Aβ toxicity. SIGMAR1 also promotes autophagy and results in the degradation of amyloid-beta precursor protein (‘APP’), thereby normalizing Aβ production (source of quote).
Much of the above would be augmented if blarcamesine also directly inhibits and reverses oxidation and nitration. That there is some limited benefit from blarcamesine even in individuals with poor sigma-1 receptor functioning suggests that it also acts as a direct antioxidant.
At 57 weeks, blarcamesine resulted in a mean improvement of .5 in MMSE scores. When patients with moderate Alzheimer’s (baseline lower than 20) and patients carrying a sigma-1 receptor mutation were removed, the scores improved to 1.7 points. Removing those with a COMT mutation resulted in a score improvement of 2.0 points (results). As Alzheimer’s progresses, sigma-1 receptor activity decreases on its own (in reaction to less intracellular calcium release), while those with sigma-1 receptor mutations have non-functioning or poorly functioning sigma-1 receptors to begin with: this limits the ability of blarcamesine to lower oxidative stress in both these sets of patients. For its part, a COMT mutation can increase oxidative stress. Blarcamesine works best in mild Alzheimer’s disease where sigma-1 receptor agonism still makes a difference and when peroxynitrite levels are still relatively low.
Anavex, thus, largely knows why certain patients respond better to blarcamesine than others. At 109 weeks, six out of 21 patients stabilized or showed improvement on blarcamesine (5 out of six had high plasma concentration levels) (results). Any improvement or stabilization at or past 18 months is a sign of modification of Alzheimer’s disease.
Cyclo Therapeutics (CYTH) TrappsolCyclo
Trappsol Cyclo inhibits peroxynitrite formation by reducing the size of lipid rafts via the removal of cholesterol (polysaccharides). The reduction of lipid rafts is critically important because the processes which lead to the onset and progression of Alzheimer’s disease take place in these rafts (and this is magnified for APOE4 carriers who have larger lipid rafts to begin with).
Lipid Rafts in Alzheimer’s Disease (International Journal of Alzheimer’s Disease)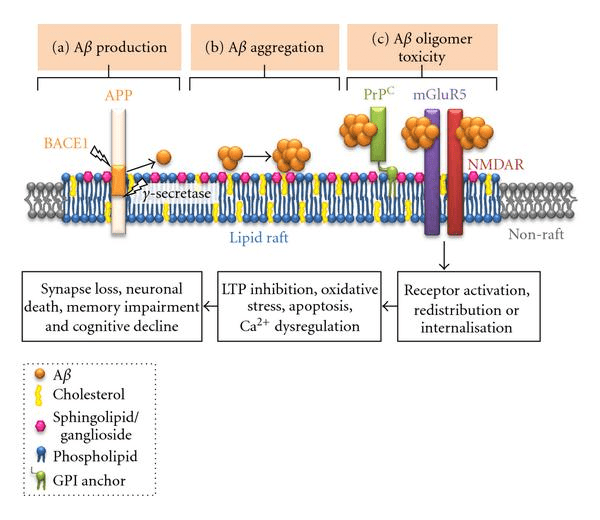
Trappsol Cyclo also scavenges peroxynitrite (polysaccharides). Currently Trappsol Cyclo has shown positive effects in one patient:
After 18 months, the patient showed neurologic and cognitive stability: this is a positive given that persons with Alzheimer’s Disease dementia are generally expected to decline during an 18-month timeframe. Speech fluency also improved as documented by the treating physician’s report of a decrease in latency to word-finding. In addition, mood and behavioral features of the disease improved, such as less agitation, as noted by the patient, the patient’s family and the treating physician (source of quote).
Despite limited resources, the company will hopefully be able to undertake and complete it phase 2a clinical trial for Alzheimer’s disease.
Panx ginseng/Korean red ginseng
Panax Ginseng (J Ginseng Res.)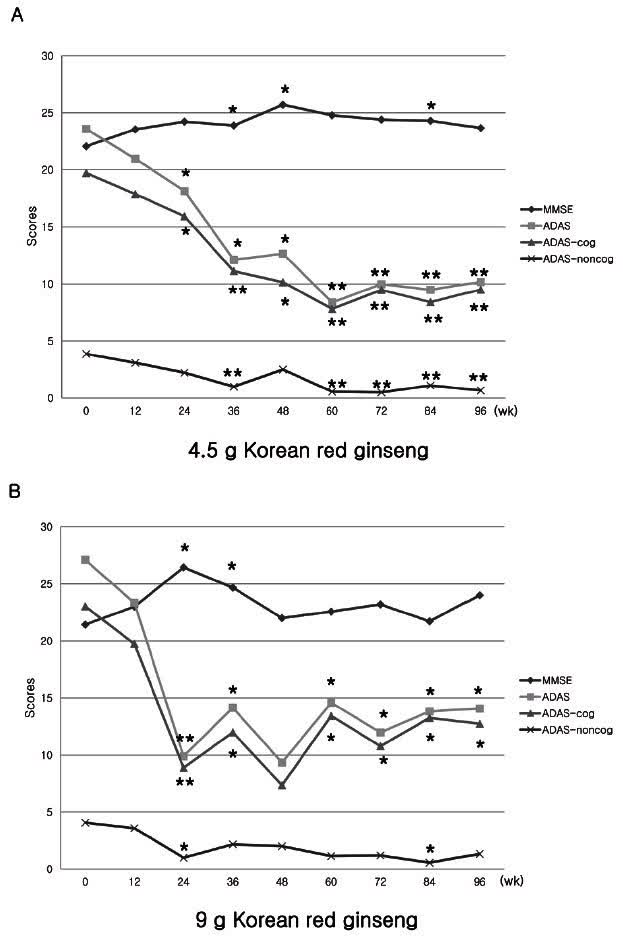
Panax ginseng inhibits peroxynitrite formation via inhibition of Nuclear factor Kappa B (polysaccharides, saponins, polyphenols). It also scavenges peroxynitrite (polysaccharides, saponins, polyphenols). The results produced by this dual action are very similar to that identified by Anavex for blarcamesine:
Panax ginseng C.A. Mey. is a well-known medicinal plant that…has neuroprotective effects against a series of pathological cascades in AD, including beta-amyloid formation, neuroinflammation, oxidative stress, and mitochondrial dysfunction (source of quote).
Panax ginseng is a natural product and faces the resistance that all natural products face for the treatment of Alzheimer’s disease, especially in the United States. Therefore it is very difficult to gain funding for trials to follow up on the ones that have taken place in South Korea (another trial).
For the time being at least, this leaves Anavex’s blarcamesine as the best current hope for the near stabilization of Alzheimer’s disease over the long-term. Results from Anavex’s 48 weeks phase 2b/3 trial may be known late in the Fall. It is possible that the company will be able to present the results at the CTAD conference in late November and early December. The pattern of early improvement in some patients that carried all the way through to 109 weeks should be replicated in a much larger trial at 48 weeks. From there even without U.S. trial centers, the FDA should grant accelerated approval, allowing for the sale of the drug, but with continued testing, with U.S. sites, over longer periods of time. Nothing is for certain, but all of this should give long-time investors in the company considerable optimism and perhaps draw a few new investors before the release of trial results.


Be the first to comment